
Holistic open learning networks offer a new drug R&D model for improving research outcomes.

Holistic open learning networks offer a new drug R&D model for improving research outcomes.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

The past three decades have driven a purchasing evolution to a procurement revolution.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.

Tracking changes from spinoffs of chemical companies to life-sciences powerhouses.
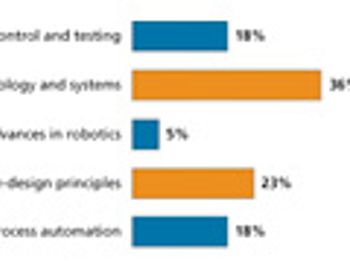
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Companies roll out expansions in manufacturing high-potency APIs and finished products.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

Advances in targeted drug delivery and customized release profiles are key goals.

Improvements in expression platforms and enhanced tools for selecting clones are among the advances of the past few decades.

Flow chemistry and microreactors offer alternatives to traditional batch manufacturing.

Uniform dose formulation is key to meeting safety study requirements.

A look back at key nanoformulation advances and what lies ahead for nanoparticle-based drug-delivery systems.

Gold nanoparticles for targeting tumor sites and elastic capsules using nanosized flakes are some recent approaches used to control and target drug delivery.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the June 2012 edition from EMD Millipore and Meissner Filtration Products.

An understanding of the pan-coating process based on first principles can support successful scale up.

Needle-free jet injection systems, gold-coated nanoparticles, and elastic capsules with nanosized flakes advance targeted drug delivery.

Peptides and related technologies to are starting to improve production.
When designing stability protocols, formulation, storage, and dosing conditions must be assessed.
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.
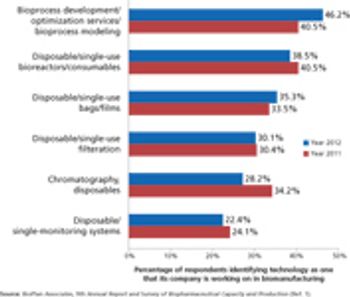
Industry wants more innovation, but can suppliers meet customer needs?

Partnerships between industry and academic medical centers are expanding to meet R&D needs.

A number of factors need to be considered when evaluating preclinical dose-formulation stability. The authors discuss formulation, storage and dosing conditions.

A technical forum featuring Tim Freeman of Freeman Technology and Carl Levoguer of Malvern Instruments.