
An uncertain regulatory environment affects funding for biotechnology.

An uncertain regulatory environment affects funding for biotechnology.
Chemocatalytic and biocatalytic routes play an important role in improving the manufacture of intermediates and active pharmaceutical ingredients.

In any industry, inspections can be a pain, and pharma is no exception.

The need for greater process understanding raises the bar for suppliers.

This technical forum is part of a special issue on Solid Dosage and Excipients.
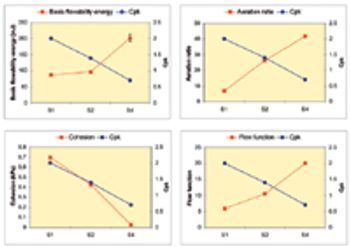
The author explains how to gain an understanding of the relationships between powder characteristics and process performance to match filling-machine geometry to the demands of specific formulations.

The author focuses on how industry can build a system for Total Excipient Control.
Representatives from Pfizer R&D, DEM Solutions, Colorcon, and ARmark Authentication Technologies provide insight into recent tablet-coating technologies.
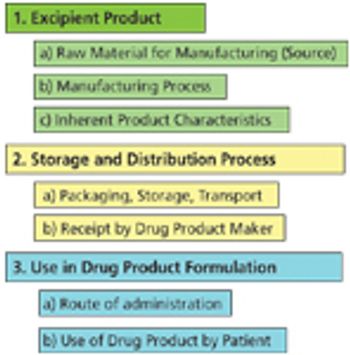
The author describes key considertions for a complete risk-assessment model and provides insight into a pending IPEC guideline in this area.

Nanotechnology often is associated with parenteral drug delivery, particularly for anticancer therapies, but it also has applications in oral drug delivery
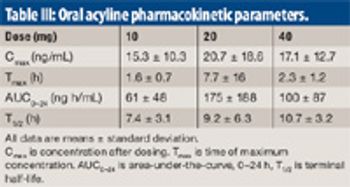
The authors examine an oral-absorption-enhancement technology based on surface-active materials to increase apical membrane fluidity in vitro.

The National Institutes of Health?s (NIH) definition of embryonic stem (ES) cells poses new challenges for investigators who seek federal research funding.

Many facilities buy compressed gas tanks or evaporate liquid nitrogen to supply processes with dry, high-purity nitrogen. An in-house nitrogen generator, however, provides several significant benefits.

Fujifilm and Merck & Co. have formed a definitive agreement by which Fujifilm will acquire the Merck BioManufacturing Network, a contract biopharmaceutical manufacturing and development business of Merck.

Pfizer Completes King Acquisition; ViiV Healthcare Names Chairman; and More.

With a regulatory pathway for follow-on biologics, industry is wondering what FDA will do next.
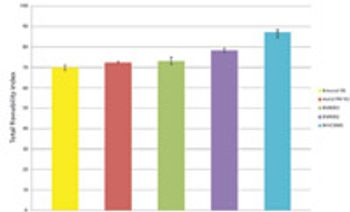
The authors investigated the tableting properties of PanExcea MHC300G, a high-performance excipient.
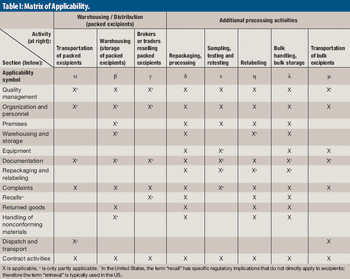
A new audit guide aims to improve supply-chain security and supplier qualification practice.
The author provides an overview of QbD implmentation for biopharmaceuticals.

Just when things seem to be looking up, the unexpected problem occurs.

The power of emerging markets is reflected in the pharma's sales and production positions.

INTERPHEX 2011 aims to address the industry's unique characteristics.

This article is Part I of a three-part series on biopharmaceutical issues in public health, government, and developing-world markets. Part 1 focuses on drug-development. Part II, which examines manufacturing, appeared in the April 2011 of Sourcing and Management. Part III, which analyzes distribution and administration,appeared in the May 2011 issue.

Contract manufacturers and fine-chemical suppliers announce capacity expansions and service enhancements of Informex.

Many solid oral dosage forms do not dissolve at the correct place in the gastrointestinal tract for them to be absorbed into the bloodstream. Could magnets solve the problem?