
The main drivers for innovation in film coatings in the last 5 years mainly relate to optimising the film coating process itself, as well as to the need for film coating materials with enhanced functionality.

The main drivers for innovation in film coatings in the last 5 years mainly relate to optimising the film coating process itself, as well as to the need for film coating materials with enhanced functionality.

Excipient purity is critical in all applications of drug formulation. Typically, the more sensitive or reactive an API, the more critical excipient purity becomes.

AstraZeneca and Abbott End Program; PhRMA Elects New Members; and More.

Pfizer Recalls One Lipitor Lot; Sanofi Aventis Names Head of R&D; and More.

Thermo Fisher to Acquire Dionex; Former SOCMA CEO Joe Acker Dies; and More.

Company and People Notes: GlaxoSmithKline acquires Nanjing MeiRui Pharma; Cephalon's CEO to remain on medical leave; and more.

Supportive public policy is needed in order for innovation to flourish.
Approaches in cyclization, palladium-catalyzed cross couplings, fluorination, and natural product synthesis help to optimize routes for select drugs.

Sometimes doing what you think is right ends up being completely and utterly wrong.

IPEC extends its reach to Brazil and Argentina in an effort to harmonize excipient best practices.
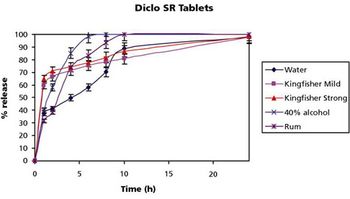
The authors evaluated the effect of alcoholic beverages on the release profiles of sustained-release dosage forms containing metformin and diclofenac.

Genzyme Sells Business Unit to Sekisui Chemical; Ablexis Names VP of Research; and More.

During the 20 months before the crisis of contaminated heparin in early 2008, the US Food and Drug Administration did not inspect any Chinese heparin firms, according to a US Government Accountability Office, (GAO) report.

Roche Details Restructuring Plan; Sigma-Aldrich Names Successors after CEO's Death; and More.

To find out about the regulatory, formulation, and manufacturing considerations involved in developing a new device to deliver a drug, Equipment and Processing Report talked to Paul Wotton, CEO of Antares Pharma.

Eli Lilly Acquires Avid Radiopharmaceuticals; EMA Recruiting New Director; and More

The market for orally disintegrating and fast dissolving tablets could exceed revenues of $13 billion by 2015 based on upward global growth trends, according to a report from Technology Catalysts International, a technology transfer and business consulting firm based in Virginia.

sanofi acquires BMP Sunstone; DCAT Names President; and More.
Editors' picks of pharmaceutical science and technology innovations.

Abuse-deterrent combination drugs represent a niche area in formulation development.

USP is working to ensure quality standards and to increase public information.

An analysis of the approaches and tools used to tackle the problem of poorly soluble drugs.

Industrial and academic partnerships forge new territory in solid-state chemistry.

As technology advances, industry's needs are growing.

Scientists and practitioners must work together for the overall good of the patient.