
The authors present three approaches that a contract development and manufacturing organization can consider when designing development and process-optimization studies that will provide useful data for scaling up a project.

The authors present three approaches that a contract development and manufacturing organization can consider when designing development and process-optimization studies that will provide useful data for scaling up a project.

Pharmaceutical Technology gains insight into approaches for producing aromatic amines.

From weekend deliveries to nonsterile gloves, a single exception can make a product fall flat.

New data provide insight into pharma-industry professionals' daily lives.

Changing demands in drug development lead to new service combinations and models.
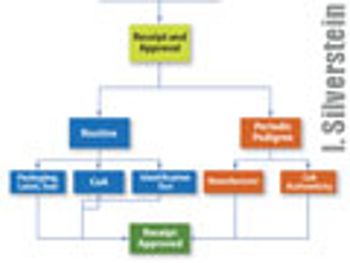
The author explains the background behind the excipient pedigree and how to implement its use.

The authors discuss a continuous-flow reactor that avoids parallel channels and enables economic plant setup. This article is part of a special issue on API Development, Formulation, Synthesis and Manufacturing.
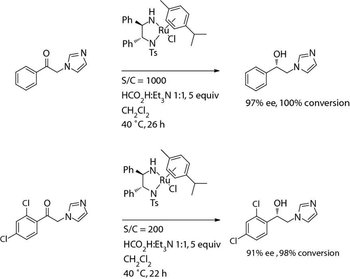
The authors describe several examples of using asymmetric hydrogenation and biocatalysis for synthesizing several secondary alcohol compounds.

One particularly crucial parameter for nasal sprays is the size of the droplets produced during actuation, which can potentially impact bioavailability.


Week of Aug. 23, 2010: Company and People Notes: Quark and Novartis Form Agreement; Hospira CEO Retires; And More.

Two new reports describe the vaccine market's recent growth and predict future market expansions.

Eli Lilly Ends Semagacestat Program; Selecta Appoints CEO; And More.

Making highly potent active pharmaceutical ingredients (HPAPIs) can be costly because the process often requires equipment specialized to achieve containment and extra attention to safety concerns. Pharmaceutical professionals may wonder whether disposable components, which have reduced the cost of some operations, might be appropriate in the manufacture of HPAPIs.

Sen. Michael Bennet (D-CO) introduced a bill earlier this month, the Drug Safety and Accountability Act of 2010, which is designed to improve regulatory oversight of drug-manufacturing facilities and improve related quality standards and monitoring.

Penwest and Endo to Merge; Watson Makes Senior Appointments; And More.

In a draft guidance published this week, the US Food and Drug Administration recommended that makers of transdermal and transmucosal drug-delivery systems use "an appropriate scientific approach" during product design, development, and manufacturing to minimize the amount of residual drug substance present at the end of the products' labeled use periods.

FDA Approves Flu Vaccines; Caraco Names COO; And More.

Industry's focus on cost cutting has led to a dangerous gap in training and knowledge.

Cases of overlooking proper packaging, reconstitution, directions, and dissolution.

FDA chemistry reviewers in the Office of Generic Drugs provide an overview of common deficiences cited throughout the Chemistry, Manufacturing, and Controls section of ANDAs.

Manufacturers and regulators on both sides of the ocean move to ensure the safety of heparin and other globally distributed drug products.

Pharmaceutical Technology talked to experts in siRNA-drug development to gain insight into the characteristics, processes, and challenges of this class of therapeutics.

Chemocatalytic and biocatalytic approaches in asymmetric synthesis help provide a pathway for producing single-enantiomer drugs.

Green chemistry in pharmaceutical development often centers around approaches in the synthesis of the active ingredient, but green-chemistry techniques also can be applied to drug-product manufacturing, formulation development, and drug delivery.