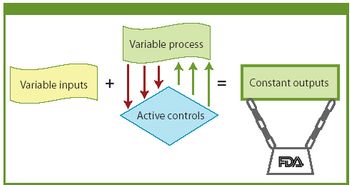
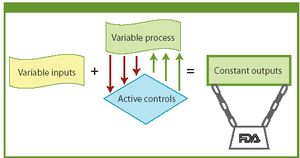
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.
Andrew G. Edwards is a senior consultant at EduQuest.
Published: February 2nd 2008 | Updated: