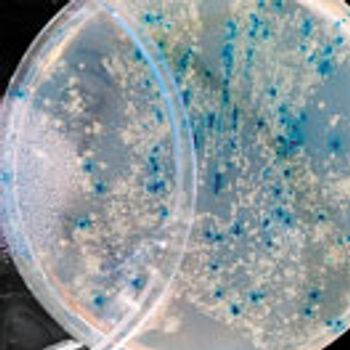
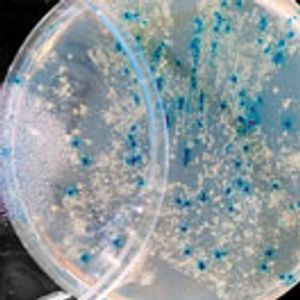
Case studies compare efficacy testing of preservatives for topical formulations with probiotic actives.
David W. Osborne, PhD, is Chief Scientific Officer and Founder, Independent Derm Solutions, LLC, 4215 Creekside Court, Fort Collins, CO 80525.

Case studies compare efficacy testing of preservatives for topical formulations with probiotic actives.

This article will clarify reasonable expectations for the responsibilities of topical product formulation developers and for excipient suppliers regarding the information and samples for experiments needed for QbD.

This article will equip the excipient vendor with an understanding of QbD from the perspective of the topical pharmaceutical product manufacturer.

This article summarizes the classification systems for topical liquid and semisolid dosage forms used for dermatological application and notes some differences between FDA and USP classification.

Published: October 2nd 2008 | Updated:

Published: October 2nd 2016 | Updated:
Published: November 2nd 2016 | Updated:
Published: March 2nd 2018 | Updated: