
The authors present the results of a survey of small- and large-molecule pharmaceutical and biopharmaceutical companies on implementation of Analytical quality by design concepts.
Elizabeth Hewitt is senior scientist I, Elizabeth.Hewitt@mpi.com,at Millennium Pharmaceuticals, Inc., 40 Landsdowne St., Cambridge, MA 02144, USA.

The authors present the results of a survey of small- and large-molecule pharmaceutical and biopharmaceutical companies on implementation of Analytical quality by design concepts.
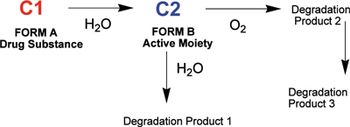
In this article, the authors demonstrate that a normal-phase chromatographic method was stability-indicating for a water-sensitive prodrug. The stress conditions using aqueous and non-aqueous conditions were also compared to understand the information obtained with each approach.
Published: March 2nd 2014 | Updated:

Published: April 2nd 2017 | Updated: