
New Age Industries, a manufacturer of plastic molding and fittings for the pharmaceutical and biopharmaceutical industries, implements a solar-energy project at its Pennsylvania facility.
See Erik Greb's bio page.

New Age Industries, a manufacturer of plastic molding and fittings for the pharmaceutical and biopharmaceutical industries, implements a solar-energy project at its Pennsylvania facility.

RUSNANO, a corporation established by the Russian government to foster the development of nanotechnology, has entered into a project to manufacture targeted-delivery nanodrugs to treat malignant neoplasms.

Drugmakers have many incentives to avoid overfilling their containers, including the scarcity, and correspondingly high cost, of certain cells and ingredients. These concerns highlight the need for techniques that can fill small volumes of product with great accuracy. Many strategies are available to the industry, but which one works best?

The FDA has unveiled a new program to improve the efficiency of import inspections.

Pfizer?s first updated pipeline since its acquisition of Wyeth includes fewer projects than before and is targeted to specific diseases; after the acquisition, the companies? combined pipeline had 600 projects, but the number has been reduced to about 500.

The World Health Organization?s (WHO) response to the H1N1 pandemic was not improperly influenced by the pharmaceutical industry, Keiji Fukuda, special adviser on pandemic influenza to the WHO?s Director General, explained at a hearing held by the Council of Europe to address concerns over the WHO?s reaction to the pandemic.

The European Public Health Alliance (EPHA) has published its comments about the Falsified Medicines Directive, which is currently under debate by the European Parliament and Council.

The Congressional Budget Office (CBO) has released a report titled Promotional Spending for Prescription Drugsthat analyses the pharmaceutical industry?s advertising strategies.

The US Pharmacopeial Convention (USP) has posted a proposed revised standard for what should and should not appear on the ferrules and cap overseals of medication vials.

The FDA is using prescription data from Wolters Kluwer Pharma Solutions to track the treatment of influenza A (H1N1) and other influenza viruses.

The FDA has published its first draft guidance for industry about Risk Evaluation and Mitigation Strategies (REMS).

Eli Lilly and Co. is undergoing a companywide reorganization intended to accelerate the progress of the company?s pipeline.
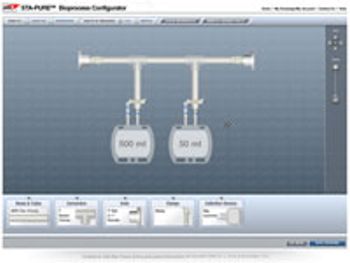
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the October 2008 edition from Cognex and W.L. Gore and Associates.
Several strategies and software applications help pharmaceutical companies integrate their manufacturing execution systems and enterprise resource planning systems.

Pharmaceutical companies want software vendors to make integrating manufacturing execution systems and enterprise resource planning systems easier.

Qualification demonstrates that equipment is what it is purported to be and does what it is supposed to do. Installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) are the three steps of equipment qualification.
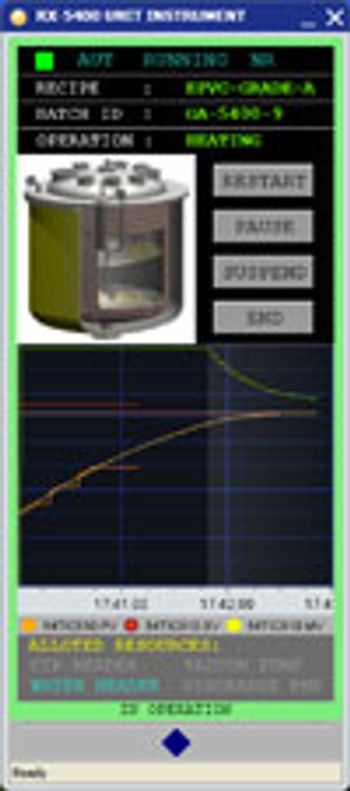
PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the September 2008 edition from LiquaJet and Yokogawa.

Although the spotlight is now on nanoparticulate delivery for biologicals, other strategies have proven successful.

Companies continue to develop inhaled insulin and other drugs, despite the problems that Pfizer's "Exubera" experienced.

What makes a drug ripe for respiratory delivery?

Featured products from this issue of Equipment & Processing Report
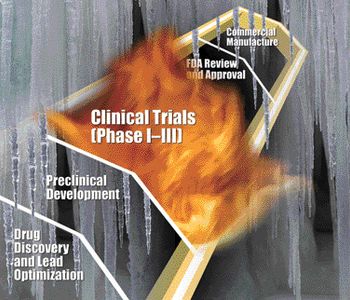
The outsourcing of clinical-trial materials grows as pharmaceutical companies adapt to a changing market.

What should contractors and their clients bear in mind when they collaborate on a project?

New York (Apr. 26)-Bristol-Myers Squibb Company and Pfizer, Inc. entered into a collaborative agreement for the development and commercialization of apixaban, an anticoagulant that BMS discovered.

Published: July 12th 2011 | Updated:

Published: February 19th 2010 | Updated:

Published: February 12th 2010 | Updated:

Published: February 17th 2010 | Updated:

Published: January 29th 2010 | Updated:

Published: February 5th 2010 | Updated: