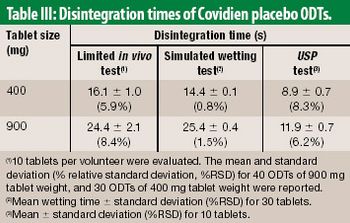
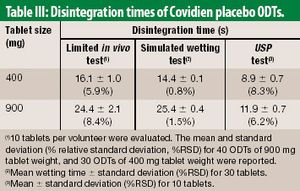
The authors propose an alternative to the USP disintegration test method. The method embraces physiological conditions of the oral cavity, as a screening tool for developing ODT products.

The authors propose an alternative to the USP disintegration test method. The method embraces physiological conditions of the oral cavity, as a screening tool for developing ODT products.
Published: August 2nd 2008 | Updated: