
Will efforts to limit all returns on investment drive biopharma companies away from developing much-needed interventions for COVID-19.

Jill Wechsler is Pharmaceutical Technology's Washington Editor, jillwechsler7@gmail.com.

Will efforts to limit all returns on investment drive biopharma companies away from developing much-needed interventions for COVID-19.

FDA and the US Congress support innovation and access to cheaper medicines.

Despite the clear danger of COVID-19 to global health, vaccine opponents have gained ground, as fearful populations lose faith in the capabilities of industry and government to protect public health.

The White House looks to bring in credible experts for its Operation Warp Speed initiative to advance therapies and vaccines to combat the coronavirus pandemic.

The Trump Administration has awarded a hefty contract to a new pharmaceutical manufacturing consortium to produce in the United States all components of certain critical medicines needed to combat COVID-19.

Biopharmaceutical companies and federal agencies have been working overtime and assuming considerable risk to be able to supply billions of doses of any safe and effective preventive.

Some observers fear that political interference in the process may erode confidence in the scientific basis for FDA regulatory decisions.

Congressional leaders are developing the next version of the 21st Century Cures Act, including provisions to advance research related to the COVID-19 crisis as part of initiatives for bringing innovative therapies to market faster.

Policy makers seek to ensure supplies of new therapies and to limit shortages.

With hundreds of clinical trials for potential coronavirus therapies in the works concerns have mounted about the emergence of conflicting data, useless results, and wasted efforts from multiple overlapping efforts.

Drug manufacturers, distributors, and dispensers oppose Buy American policies as likely to reduce reliable supplies and raise product costs.

FDA is encouraging alternative insulins and challenging anticompetitive practices.

The bill includes multiple less-noticed provisions to bolster healthcare programs and to advance the development of new treatments and preventives to combat the virus.

FDA officials are rolling out guidance and support for researchers striving to assess potential treatments for COVID-19 while the agency tries to object to premature optimism and regain public credibility.

FDA is offering advice and added flexibility to help sponsors adjust ongoing and planned clinical research programs during the COVID-19 outbreak.

States, hospitals, and insurers support manufacturing arrangements to ensure access to affordable medicines.

US and European regulatory officials continue to anticipate supply shortages in multiple areas.

The research community is moving quickly to launch clinical trials of potential countermeasures, while regulatory authorities aim to support product development through regulatory flexibility.

The FDA Commissioner plans to address drug prices, the drug approval process, and supply chain issues during his time as commissioner of FDA.

Increased reliance on foreign producers raises concerns and spurs collaborations.

Top of CDER’s to-do list for 2020 is tracking adverse events more effectively and combating the opioid crisis.

Can a commitment to limit prices to ensure patient access to important new medicines regain public trust and confidence in the biopharmaceutical industry?

The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.

Pressures on FDA will affect industry’s success in bringing new therapies to market.

While labor and tariff reforms in the revised North American trade agreement may have more visible impacts on the United States economy, the final document levels a major blow to exclusivity and patent protections important to innovator biotech and pharma companies.

FDA has an official new leader, following a Senate vote to confirm Stephen Hahn as the agency’s next commissioner.
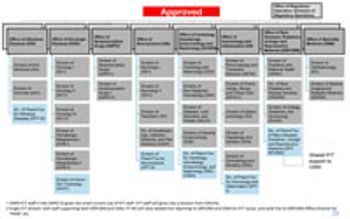
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

Developers of biosimilars have become dismayed with difficulties in gaining acceptance and reimbursement from the US healthcare system.

FDA readies more efficient oversight processes while advancing collaboration with Europe.