
Although new single-use technologies offer the flexibility needed to overcome several challenges in ATMP production, there are many considerations and hurdles manufacturers must be aware of when scaling.
Kai Lipinski, PhD, is the chief scientific officer of Vibalogics.

Although new single-use technologies offer the flexibility needed to overcome several challenges in ATMP production, there are many considerations and hurdles manufacturers must be aware of when scaling.
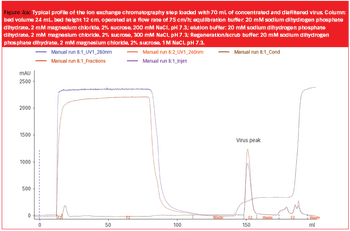
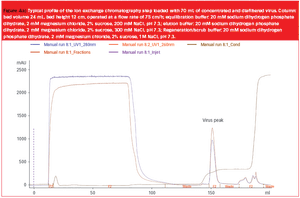
The use of viral vectors as gene delivery and vaccine vehicles has developed rapidly during the last two decades owing to several viral properties. Viruses can infect cells efficiently, often have a broad tissue tropism and can achieve very high levels of either stable or transient transgene expression. Furthermore, their intrinsic immune-stimulatory properties can have adjuvant effects during the treatment of cancer or infectious disease and, importantly for manufacturing scale-up, some viruses can be grown to very high titre (.1012 particles/mL). The development of robust production procedures is essential to move therapeutics that utilize viral vectors into clinical trials, and to make them cost effective for market supply. Here, we describe some of the aspects of production that must be considered and optimized when producing virus vectors on an intermediate or large scale. By drawing examples from our experience of vector production, we show that upstream and downstream processes must be designed..

Published: February 15th 2023 | Updated:
Published: January 1st 2008 | Updated: