
New FDA Guidance on Electronic Common Technical Document Submissions
Laura Bush is a former managing editor of Pharmaceutical Technology and Editor-in-Chief of BioPharm International.

New FDA Guidance on Electronic Common Technical Document Submissions

Oddly for a technical meeting, the Oct. 5–7 AAPS Workshop on Pharmaceutical Quality Assessment focused on words, with an entire session devoted to definitions and countless discussions of meaning and nuance.

Collaboration Targets Single-Shot Japanese Encephalitis Vaccine

For years, pharmaceutical manufacturers have complained that federal regulations impede process improvements. Now, FDA is trying to do something about it.

The translator strives for quality, for a flawless re-setting of ideas from language to language. It is precise, painstaking work, making sure that all the concepts are there, that the grammar and syntax are correct, that the register (the formality, the emotional and social tone) is accurate, and that the text reads smoothly.
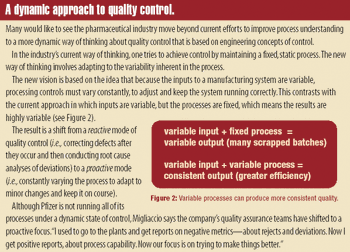
In 1987, when the US Food and Drug Administration issued its Guideline on General Principles of Process Validation, a young FDA reviewer asked her supervisors.

Although the United States Pharmacopeia-National Formulary is an everyday reference book, the people and processes behind it are not so familiar.

As FDA prepares for the Critical Path initiative, offices across the agency are expanding the use of the risk-based approach to regulation and applying a quality systems approach to internal operations.

Published: November 3rd 2005 | Updated:

Published: November 2nd 2005 | Updated:

Published: October 2nd 2005 | Updated:
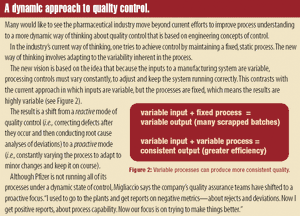
Published: August 2nd 2005 | Updated:

Published: October 2nd 2005 | Updated:

Published: October 6th 2005 | Updated: