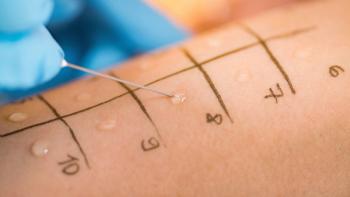
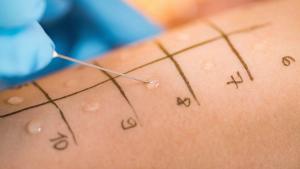
FDA’s new guidance on in-vitro permeation test studies published in October 2022 gives technical and statistical requirements for conducting these tests to compare topical generic drugs with their reference products.
Lei Lei is senior R&D director of Cutia Therapeutics, Shanghai, China, and he was principal scientist of Shanghai Johnson & Johnson Pharmaceutical Company

FDA’s new guidance on in-vitro permeation test studies published in October 2022 gives technical and statistical requirements for conducting these tests to compare topical generic drugs with their reference products.
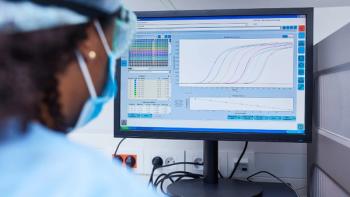
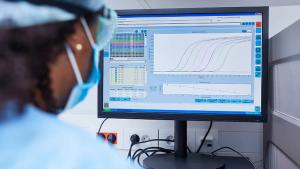
This article describes the in-vitro permeation test study data processing procedures and FDA statistical mathematics of evaluating a generic topical drug product, acyclovir cream, against its reference product.

The CuDAL-Excel program, based on Microsoft (MS) Excel, has been developed to calculate the United States Pharmacopeia (USP) passing probability of content uniformity and dissolution tests for both sampling plan 1 and sampling plan 2 scenarios and for both immediate release and extended release requirements. The users can obtain the passing probability by simply entering the input variables, with wide applications for process validation/verification and batch release. As a user-friendly program, CuDAL‑Excel should bring more benefits to the industry practitioners than other existing programs/tools.
Published: June 3rd 2022 | Updated:
Published: July 3rd 2023 | Updated:

Published: May 2nd 2020 | Updated: