
The agency has recommended granting marketing authorization for Opdivo.

The agency has recommended granting marketing authorization for Opdivo.

Dicerna Pharmaceuticals announces that FDA granted its primary hyperoxaluria type 1 (PH1) treatment Orphan Drug designation.

The company voluntarily recalls Preservative-Free Bupivacaine HCl Injection, USP due to potential iron oxide particulates.
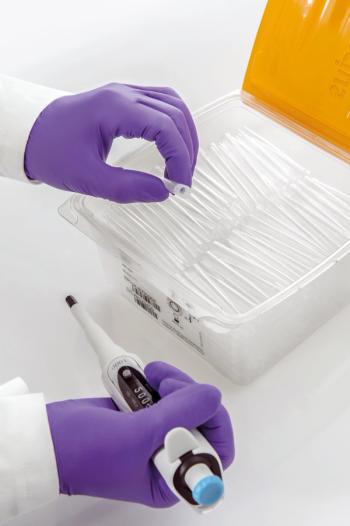
FlexiBulk tip packs save space and effort in the laboratory

MaxCyte announces a strategic partnership with Johns Hopkins University to develop CAR T-Cell therapies for cancer.

INTERPHEX 2015 is under way and Pharmaceutical Technology and BioPharm International are in the middle of the action!

With the inauguration of the Technology Center, the engineers, scientists, pharmacists, and IT developers for the first time closed the loop control circuit of a modular system along the entire process including production, sensor technology and controlling.

The agency has released draft guidance on providing regulatory submissions in regards to promotional labeling and advertising materials.

FDA grants Pfizer Breakthrough Therapy designation for its treatment of ROS1-positive non-small cell lung cancer.

Teva announces that it would pay up to $40 billion in cash and stock to acquire Mylan.

The European Medicines Agency releases findings from marketing authorization application analysis.

The combination of two mAb drugs eradicated a large tumor, but also sparked new toxicity concerns associated with immunotherapies.

GSK notifies CDC and FDA that it is recalling the remaining doses of its 2014–2015 flu vaccine, Flulaval Quadrivalent Thimerosal-free Pre-Filled Syringes, due to decreased potency.

Catalent completes a $52 million expansion program for advanced oral solid manufacturing solutions at its Winchester, KY, facility.

The acquisition expands Sartorius Stedim Biotech’s service portfolio.

Rentschler Biotechnologie expands European manufacturing capabilities with GE Healthcare Life Sciences bioprocess technologies.

FDA extends ANDA rule comment period to June 8, 2015 after requests for more time.

The Ph. Eur. contemplates adding specifications for sub-visible particles in eye drops and eye lotions to its monograph.

At the request of FDA, US Marshals seize unapproved prescription drugs from Florida distributor.

India may go to the World Trade Organization if the EU does not reconsider its decision to suspend the sale of about 700 generic drugs that were approved based on clinical trials by GVK Biosciences.

Recipharm makes a strategic investment in Synthonics and partners in development of novel compounds.

Pfizer announces that its Phase III trial for Ibrance met its primary endpoint and was ended early due to efficacy based on an assessment by an independent Data Monitoring Committee.

The document serves as guidance for the pharmaceutical industry in data integrity issues and complements existing EU GMP relating to APIs and dosage forms.

The single-use clarification system eliminates centrigues; harvesting can be performed in one step; and process robustness and predictability are ensured.

Japanese pharma market evolves towards generics and innovative products, reports CPhI, as CPhI Japan 2015 commences.

Hydra Biosciences enters into an agreement with Boehringer Ingelheim to research and develop small-molecule TRP inhibitors for renal diseases and disorders.

The European Medicines Agency releases guidelines for addressing and reporting risks associated with medication errors.

The license approval marks another milestone in Aptar Pharma’s ongoing service development to provide large-scale production services to customers.

The pharmaceutical market in Japan is moving towards generic and innovative products, and is expected to hit US$166 billion by 2023.

Agency teams work together to encourage manufacturers to seek approval for previously unapproved drugs.