
A new book explains process analytical technology, drug stability, and quality.

A new book explains process analytical technology, drug stability, and quality.
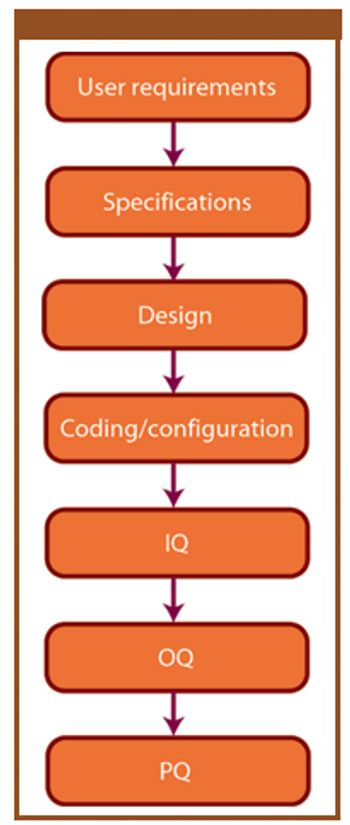
This article provides a historical review of computer validation in the pharmaceutical industry within the last three decades, evolving from the early years' initial concept and approach to today's current practices. Also included is how the regulations and industry have progressed in addressing the topic of computer validation.

Part I of this article was published in the March 2003 issue of 21 CFR Part 11: Compliance and Beyond. In this issue, Part II discusses the potential advances and changes that must be made for computer validation to remain innovative and relevant to the industry.

The authors forecast how the current industry events and trends will affect computer validation in the future.

Current events and trends in regulations, business practices, and technology forecast the future of computer validation.
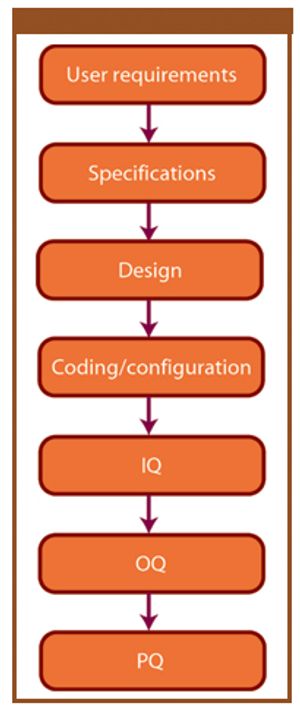
Published: July 2nd 2007 | Updated:

Published: July 2nd 2008 | Updated:

Published: July 1st 2001 | Updated:

Published: September 2nd 2001 | Updated:

Published: September 1st 2003 | Updated: