
An annual survey on inspections and audits has revealed opportunities to use more flexible approaches to optimize processes.

An annual survey on inspections and audits has revealed opportunities to use more flexible approaches to optimize processes.

The authors look at challenges and considerations to continuously improve inspection efficiency.
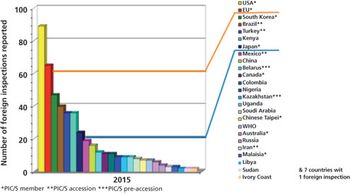
The European Federation of Pharmaceutical Industries and Associations (EFPIA) has conducted an annual survey of GMP/GDP inspections at sites and affiliates among its member companies from 2003 until the present time. This article describes findings of the EFPIA annual survey of site inspections among its member companies.


Using a model quality risk-management process according to ICH Q9, the authors discuss ways to apply this guideline.

Published: November 2nd 2021 | Updated:

Published: September 1st 2014 | Updated:

Published: August 2nd 2015 | Updated:
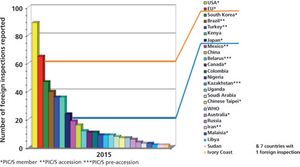
Published: January 2nd 2017 | Updated:

Published: February 2nd 2017 | Updated: