Ensuring reliable analytical methods and bioassays requires a well-thought-out strategy for evaluating method validity and systems suitability.
Thomas A. Little is president, Thomas A. Little Consulting and BioAssay Sciences, 12401 North Wildflower Lane, Highland, Utah 84003, USA, drlittle@dr-tom.com.

Ensuring reliable analytical methods and bioassays requires a well-thought-out strategy for evaluating method validity and systems suitability.
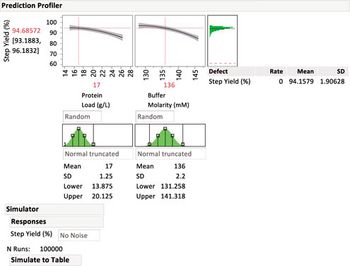
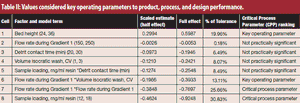
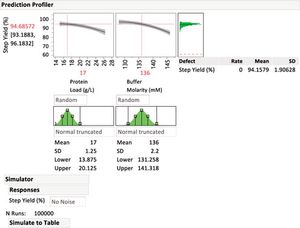
To protect drug product and drug substances, statistical methods to identify critical process parameters and critical material attributes, as well as and approaches to control them, are needed.
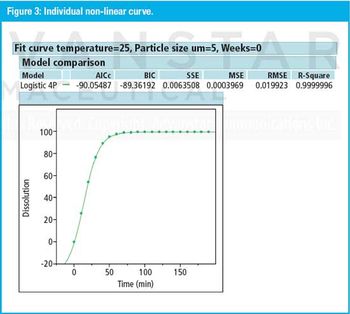
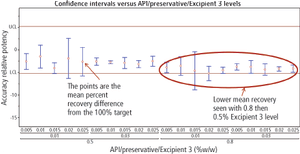
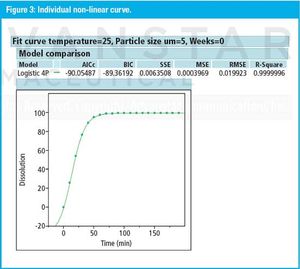
The right approach can provide a clear, statistically defendable method for determining dissolution and accelerated stability.
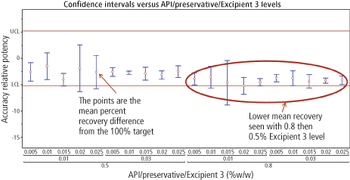
The authors describe a method-validation-by-design (MVbD) approach to validate a method over a range of formulations using both design-of-experiment and quality-by-design principles to define a design space that allows for formulation changes without revalidation.
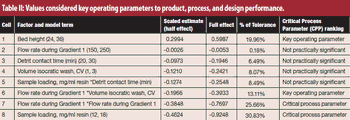
Critical process parameters (CPPs) and their associated process controls are crucial to drug development and process validation and to the evaluation of every manufacturing unit operation.
Published: February 1st 2022 | Updated:
Published: April 2nd 2013 | Updated:
Published: November 1st 2012 | Updated:
Published: July 2nd 2014 | Updated:
Published: April 4th 2018 | Updated: