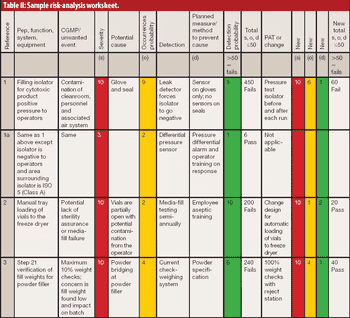
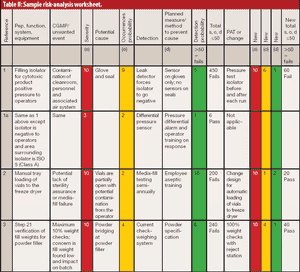
The author reviews the draft guidance on process validation, its QbD applications, and its potential impact on sterile manufacturing operations.
Warren Charlton is a consultant at WHC Bio Pharma Technical Services, PO Box 20309, Greenville, NC 27858, tel. 252.327.4733, fax 252.756.4733.
Published: May 1st 2009 | Updated: