Pharmaceutical Technology Europe
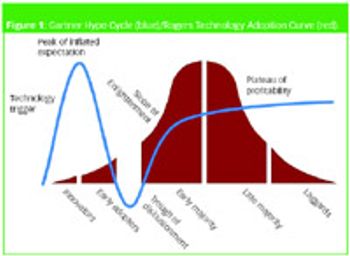
A number of commentators during the past few years have speculated that nanotechnology is the wave of the future in biotech and pharma.

Pharmaceutical Technology Europe
A number of commentators during the past few years have speculated that nanotechnology is the wave of the future in biotech and pharma.

Pharmaceutical Technology Europe
Traditionally, in pharmaceutical water systems the main method for controlling bacterial levels was heat or chemical sanitants...

Pharmaceutical Technology Europe
Drug companies have come to realize that spending heavily on creating new blockbuster drugs is risky and less cost-effective ...
Pharmaceutical Technology Europe

Pharmaceutical Technology Europe
Driven by a rapidly ageing population and a known high consumption of pharmaceuticals, the French pharmaceutical market has always appeared buoyant.

Pharmaceutical Technology Europe
... the biotech industry could make characterization of its products easier by paying more attention to downstream processing and purification issues, creating a cleaner product that is easier to identify.

Pharmaceutical Technology Europe
Anyone familiar with the USA v Barr Laboratories case of 1993 will recognize the importance of not ignoring OOS results, and the inappropriateness of testing into compliance.

Pharmaceutical Technology Europe
Vendors who insist on using older and outdated technologies will eventually suffer increased pressure to follow the lead of those who adopt new technologies.
Pharmaceutical Technology Europe
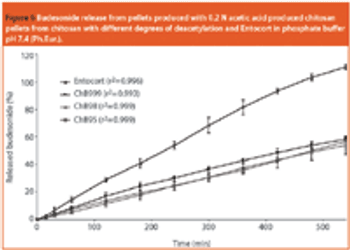
Chitosan is of pharmaceutical interest because of its positive attributes with respect to toxicity, biocompatibility and bioavailability.