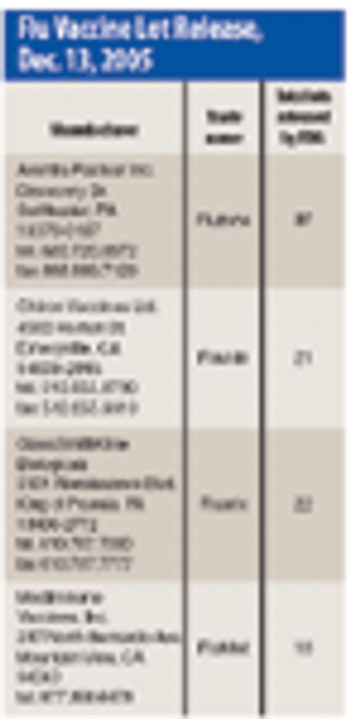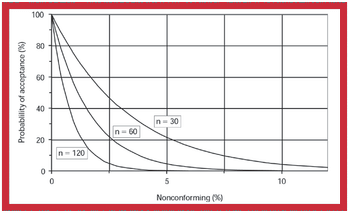
The benefits of zero tolerance as a test criterion have been oversold. A critical examination of zero tolerance reveals that many of the supposed benefits are not attainable. More important, inappropriate application of this criterion can have a deleterious effect on the assessment, control, and improvement of the quality of pharmaceutical products.