
The authors designed an upper punch with a removable punch tip to determine a tablet formulation's propensity to stick by weighing the mass of powder adhered to the punch tip.

The authors designed an upper punch with a removable punch tip to determine a tablet formulation's propensity to stick by weighing the mass of powder adhered to the punch tip.

The benefits of harmonization may be on industry's wish list, but buying into change is another story.

Recent legal decisions have further divided generic and brand manufacturer cases.

Challenges remain, particularly for early-stage biopharmaceutical companies.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.

As part of the BRIC bloc with Russia, India, and China, Brazil is one of the world's leading emerging economies and is also considered by IMS Health to be one of seven pharmerging nations, which also include Mexico, Turkey, and South Korea.

Technology may expedite operations, but the absence of the human element could cost dearly.
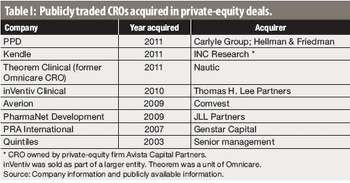
Some recent private-equity buyouts of CROs show both the upside and downside for investors.

To keep moving forward, the Pharmacopoeial Discussion Group needs industry participation.

Packaging innovations boost productivity, meet regulatory requirements, and protect products.

Some recent advances involve strategies for accelerating reaction discovery, approaches for inducing chirality and stereochemical analysis, and applications in nanotechnology for protein elucidation.

The European Union market takes steps toward continuous processing and modular facilities.

Copay coupons may help patients and drugmakers, but who ends up holding the bag?
Industry experts discuss formulation and technical challenges in multilayer tablet manufacture.

Creating a successful antibody-drug conjugate requires careful selection of the drug, antibody, and linker.

Nanosized systems are important in drug delivery. Such nanosized systems include liposomes, nanocrystals, micelles, colloidal particles, quantum dots, and dendrimers. Dendrimers are class of synthetic macromolecules with highly branched, monodispersed, circular, and symmetrical architecture that are used as carrier molecules in drug delivery.

The author describes how providing appropriate information about the API in the Common Technical Document can aid FDA's review of an abbreviated new drug application.

A Q&A with Gilles Cottier, president of SAFC, on recent industry trends.

New product reviews for January 2012.

Click the title above to open the Pharmaceutical Technology January 2012 issue in an interactive PDF format.