Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

The bio/pharmaceutical industry will face increased scrutiny of product quality and cost drivers.
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.
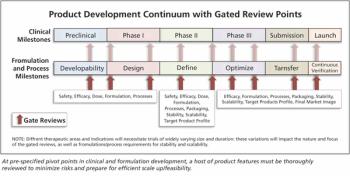
Appropriate use of sound science applied at critical junctures will improve efficiency in the high-wire act of drug development.

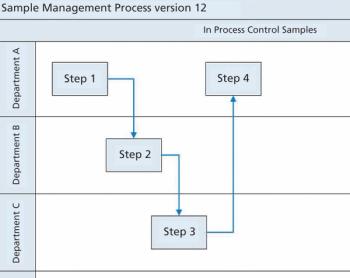
The author discusses the collection and evaluation of data part of FDA’s definition of process validation.

DAVID LEAHY/GETTY IMAGESThe pharmaceutical industry has an important role to play in implementing solutions to global envi
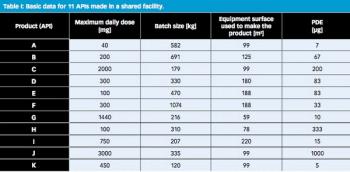
The 10-ppm criterion for the acceptable concentration of potential API in cleaning validation to minimize cross contamination into next product has been employed for many years. This article describes why the 10-ppm criterion, which was established based on analytical limitations and estimates of acceptability, is no longer necessary and why a risk-based approach should be universally adopted.

The revised USP Chapter 1207 gives best practices for obtaining reliable data in container closure integrity testing.

Industry experts discuss what the outsourcing market holds for 2016.

Infrastructure and payer decisions will determine drug choices in emerging and developed regions.
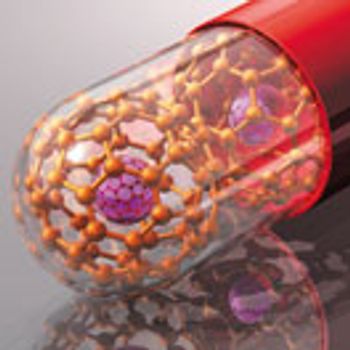
Nanoscale catalysts and engineered nanoparticles may have a big impact on small-molecule pharmaceuticals.

Amid corporate restructurings, regulatory initiatives, and aging R&D assets, will drug development accelerate or stall in 2016?

FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

Regulatory, corporate restructuring, and manufacturing issues will challenge bio/pharma to meet the needs and expectations of patients around the world.

Click the title above to open the Pharmaceutical Technology January 2016 issue in an interactive PDF format.

Leading nations are backing moves to strengthen WHO’s central role in international health security following the Ebola crisis, which sparked criticisms on the organization’s ability to address a pandemic outbreak.

Liquid formulations in hard-shell capsules or softgels are becoming a popular option for HPAPIs because of advantages such as improved safety and lower risk of potential exposure and product cross contamination.

The Prominence-i LC-2030 LT is a new model of Shimadzu’s i-Series integrated high-performance liquid chromatograph and ultra high-performance liquid chromatograph systems.

The Ross AMK Kneader Extruder combines the technologies of a double-arm mixer with an extrusion screw.

The BioClamp Plastic Tri-Clamp, from BioPure Technology, part of the Watson-Marlow Fluid Technology Group, is a patented plastic union tri-clamp meant to reduce distortion on polymeric fittings when subjected to heat.

The DF30Plus, a new version of Aptar Pharma’s aerosol metering valve for pressurized metered dose inhalers (pMDIs), incorporates an elastomeric cyclic-olefin-copolymer (COC) neck gasket.

Traceability and transparency will remain elusive if manufacturers continue to approach serialization projects on a case-by-case basis.

Synthetic biology promises to drive tomorrow’s therapies, while continuous processing is already being used in some new drugs.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.