
FDA is opening the door to continuous learning in process development.

FDA is opening the door to continuous learning in process development.
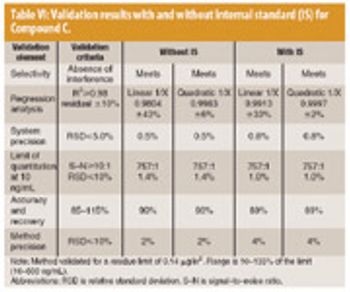
LC–MS–MS has the potential to become a routine technique for determining drug residues in support of cleaning verification, especially early in drug development.
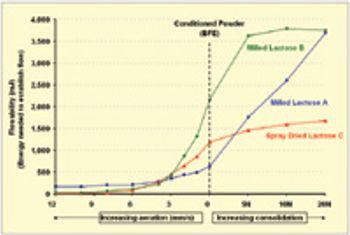
The pharmaceutical industry's focus on process understanding, monitoring, and control is driving manufacturers to take greater steps toward identifying possible manufacturing bottlenecks earlier in the development process. For tablet, capsule, and excipient producers, such efforts include taking a closer look at the flow-ability of their powders.

"It was the late 1970s," reports our GMP-Agent-in-Place, "and we used a primitive desktop computer with built-in teletype for our quality control work.

I rarely talk science on the weekends. I spend a good deal of that free time with friends from college performance groups. The conversation centers more on choreography and piano concerti than on CGMPs and chemical reactions. So it always throws me for a loop when these theater and music buffs drop pharmaceutical news into casual conversation.
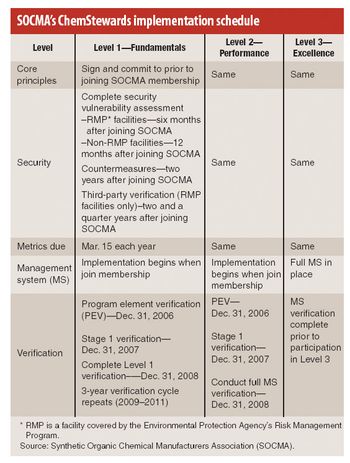
This is a year of change for the Synthetic Organic Chemical Manufacturers Association (SOCMA), the Washington, DC-based trade association representing chemical batch and custom manufacturers. Following the sale of Informex, its flagship trade show, last fall, the association is advancing key programs, most notably its new ChemStewards program, an environmental, health, safety, and security initiative (EHS&S) that its members began implementing last month.

Enterprise information integration delivers needed real-time capability to operational business intelligence.

FDA is proposing revisions to the drug user fee program while it weighs changes for drug safety initiatives and expanded vaccine production.
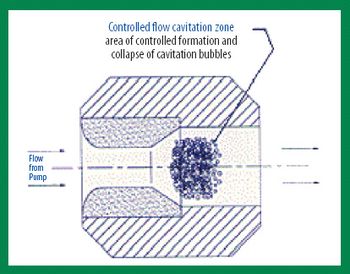
The authors present an overview of various processes for producing nanoparticles and commercial nanoparticle technologies involved in the delivery of poorly water-soluble drugs.

Preliminary results from a clinical trial of Sanofi Pasteur's (Lyon, France, www.sanofi.com) H5N1 prepandemic influenza vaccine indicate the vaccine is safe and was well-tolerated in 300 healthy volunteers. This study is the first trial of an H5N1 prepandemic influenza vaccine candidate that compared vaccines with and without adjuvants.
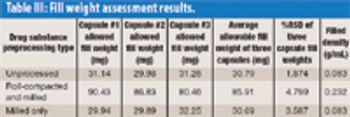
Using a novel automated microfilling system, the authors demonstrate that roller compaction followed by milling is a viable preprocessing technique for high-dose chemical-in-capsule dosage forms. The process results in higher bulk and tapped densities for drug substances compared with milling alone.

CMOs face market realities and exit some businesses.