
States, hospitals, and insurers support manufacturing arrangements to ensure access to affordable medicines.

States, hospitals, and insurers support manufacturing arrangements to ensure access to affordable medicines.

Emergency action to protect patients and the drug supply may have long-term implications.

No matter why change may be needed, it is important to comply with all the relevant regulatory requirements, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

The Double Planetary Mixer from Ross is scalable through 1000-gallon production models and is offered in a sanitary turnkey configuration that features a stainless-steel workbench for the mixer and discharge system.

The SAS Tri-Clover Isolator Head from Cherwell Laboratories allows for precise and flexible monitoring of isolator cabinets and filling lines in cleanroom, isolator, and other controlled areas.

Thermo Fisher Scientific launched the ALPS 5000 Plate Sealer to simplify the plate sealing process and provide enhanced dependability and productivity for stand-alone and integrated robotic projects.
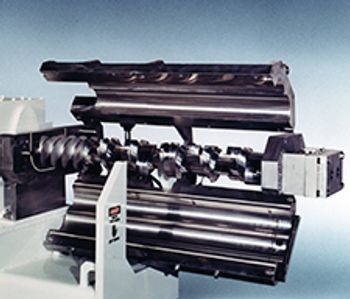
Readco Kurimoto introduced a continuous processor with a double clamshell barrel design that provides full access to the paddle and screw assembly to enable thorough cleaning and inspection processes while allowing for easy adjustments to the configuration.

Understanding of scale-up parameters and use of process analytical technology are important to meet demand for larger batch sizes.

As compounds become more complex in nature and biological ingredients are more widely used, stability testing approaches must follow suit and provide flexibility for developers.

Assays can provide a useful tool in determining the potential toxicity of drugs throughout the development cycle.

A brief overview of three notable cases of adverse drug reactions.

A study finds a potential risk of persistent visual side-effects in male patients taking the highest recommended dose of sildenafil.

Despite increasing R&D budgets by bio/pharma companies, returns on this investment are reducing and the cost of bringing an asset to market is increasing.

EU regulators have accelerated their efforts to use the mass of data emerging from the lifecycles of drugs as an effective basis for both the development and control of medicines.

Metrology has the potential to not only prevent harm to patients but also to support innovative therapeutic options.

While new industry guidance documents issued by FDA speak to the agency’s efforts to promote the development of new gene therapies, certain hurdles remain to challenge stakeholders.

Tackling process development early on can better optimize manufacturing processes for emerging therapies.
The growing interest in developing cell and gene therapies has prompted industry investment to grow manufacturing capacity.

The last year has seen intense investment activity into raising cell and gene therapy manufacturing capacity.

Energy and water waste can be improved with new technologies, and sustainability can be considered in the design of new facilities.

Can investing in early formulation studies drive a new therapy successfully across the commercialization finish line?

Formulating fixed-dose combination drugs proves more complex than simply adding one ingredient to another.

The authors present a simple-to-use Microsoft Excel-based statistical tool that uses cumulative sum techniques to aid retrospective understanding of data trends.

Click the title above to open the Pharmaceutical Technology March 2020 issue in an interactive PDF format.