
Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.
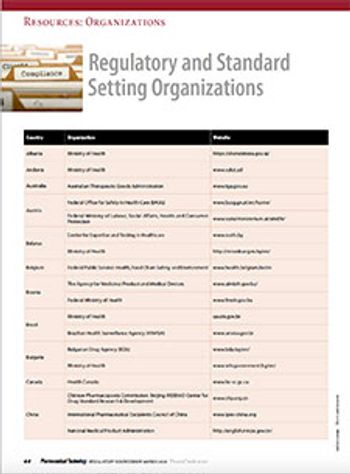
Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

With some FDA inspections on hold, will the US drug supply maintain its quality standards?

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article returns to the topic of complying with pharmacopoeial requirements with a case study at the intersection of monograph development and compliance.

In January 2020, the agency finalized six clinical development and manufacturing guidance documents and drafted new guidance on what would qualify new gene therapies as orphan drugs.

Data supporting the quality and safety of product must meet the ALCOA+ elements in order to avoid regulatory citations for data integrity issues.