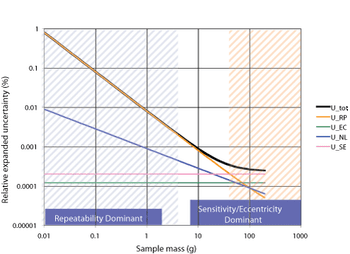
A program for calibration and routine testing of weighing instruments ensures accurate results.

A program for calibration and routine testing of weighing instruments ensures accurate results.
Science-based software and testing services expedite the material selection process and ensure blister packs deliver adequate barrier protection for solid-dosage forms without over-packaging.

Transferring the manufacturing of a drug from one scale to another or between manufacturing sites presents both technical and business challenges.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss requirements for successful product technology transfer.

Industry experts and FDA’s Office of Pharmaceutical Quality discuss the challenges, trends, and regulations involved in ensuring quality in solid and semi-solid dosage forms.
Manufacturing highly toxic compounds in a biopharmaceutical environment tests equipment and systems.

Understanding of the risks associated with FMEA is crucial in lot release testing.

A global API marketplace increases the burden of supply chain monitoring for drug companies.

Technology is making it easier to stop problems before they can affect patients and the bottom line.
Choosing the right container and container closure system is crucial for ensuring product quality, safety, and efficacy of a biologic formulation.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.

The authors discuss the concept design of a versatile, sustainable, small-scale facility in Malta that conforms to the European Union’s current good manufacturing practices.
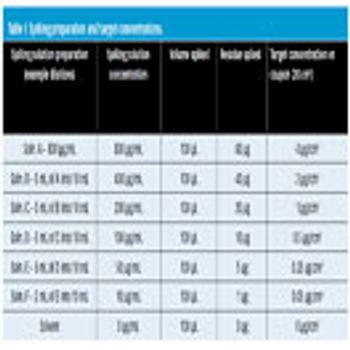
Visible residue limits have been shown to be a valuable tool in validated cleaning validation program.

Global outbreaks energize vaccine R&D and drive production modernization.

Demand is driving expansion and consolidation of formulation and clinical trial materials services.

Ross VersaMix Multi-Shaft Mixers are designed for sanitary and high-purity applications.

Peristaltic cased pump for upstream and downstream biopharmaceutical applications from Watson Marlow Fluid Technology Group.

The Anritsu Capsule Checkweigher from MG America achieves high throughput of up to 230,000 capsules per hour.
The Flexi-Cap Protect from Schreiner MediPharm.

Pharmaceutical Technology spoke with FDA to get the agency’s insights on how the industry can ensure quality in solid and semi-solid dosage products.

Click the title above to open the Pharmaceutical Technology April 2016 issue in an interactive PDF format.

A strengths, weaknesses, opportunities, and threats analysis.