
Bioprocessing and Sterile Manufacturing

Bioprocessing and Sterile Manufacturing
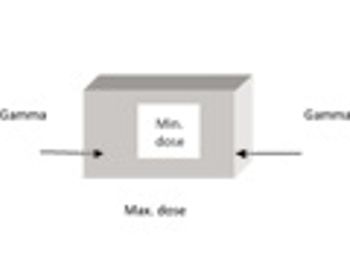
This paper examines the process of gamma irradiation of plastic materials used as part of single-use disposable systems in the pharmaceutical and biotechnology sectors, with a focus on validation requirements.
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

Parametric release and real-time testing use manufacturing data to ensure that products are made according to defined standards. PharmTech talks to Boehringer Ingelheim's Heribert Hausler about these issues.
The authors assert that the current gulf between aseptic processing and terminal sterilization can be bridged by re-examining fundamental regulatory philosophies for sterile-product manufacturing.

Closed-vial technology is an alternative to traditional glass vial filling that reduces the risk of contamination for the patient, simplifies the filling process, and provides easier handling for healthcare providers.

The authors provide a review of test methodology and standards, including current industry and regulatory proposals, for biological indicator growout times.
Readers share their views on bioprocessing challenges, equipment use, and outsourcing trends in our annual bioprocessing equipment and processing survey.