
Breaking up is easy to do.

Breaking up is easy to do.

Preferred models for clinical and nonclinical services move in opposite directions.

USP exercises a vigorous standards-setting process that draws on both public comments and meticulous scientific review.

A recent benchmarking report on pharmaceutical manufacturing raises questions about the performance of contract manufacturers, but further analysis also raises concerns about the process and data used to arrive at this conclusion.

Fewer field offices and inspectors will increase reliance on manufacturers to ensure product and process quality.

Mixed-mode chromatography sorbents and custom ligands aim to optimize protein purification.
Sanofi to Close Irish Plant, FDA Inspections and Warning Letters Continue to Decline, FDA Submits Final Proposals for PDUFA IV, and more.

Excipients facilitate formulation design and perform a wide range of functions to obtain desired properties for the finished drug product. The article reviews excipient development and functionality of these materials, including their importance in formulation design, potential processing challenges directly related to excipients, and therapeutic benefits.

The pharmaceutical majors build their capabilities in peptide technology, and contract manufacturers expand to meet growing demand for bulk peptides.
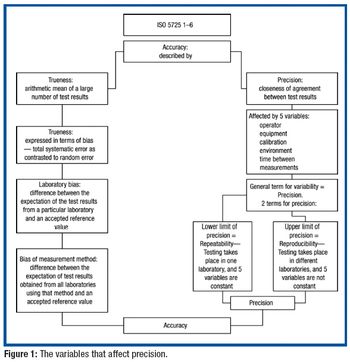
USP applies metrological principles to the dissolution procedure alone and in collaborative studies to understand and minimize potential sources of variability.