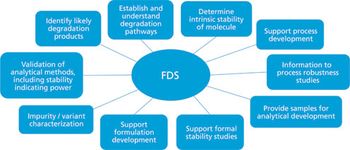
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.

The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.
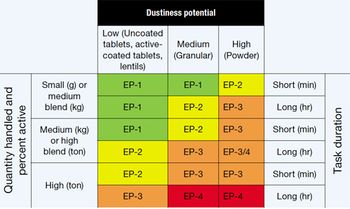
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Experts discuss some of the emerging trends in bioprocessing in 2016, including 4D bioprinting, 2D-NMR, and the CAR-T design space.
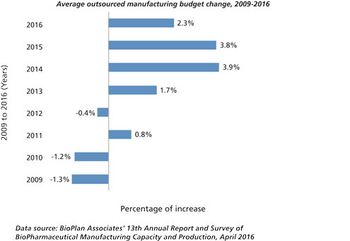
Growth may be slowing, but outsourcing activity remains healthy.

As the pharmaceutical industry moves toward continuous manufacturing, new documents are needed to provide flexible guidance that meets the new process requirements.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.

Revisions and updates for the 9th Edition of the European Pharmacopoeia result in changes to more than half of the content.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

Policies for patient access to life-saving therapies must keep pace with biomedical innovation.

Troubleshooting and collaboration are essential in implementing commercial lyophilization processes.

Application for Sirdupla Uniformity of Delivered Dose Methodology

With deadlines only a few years away, some companies have not started serialization programs, while others are taking a tactical, short-term approach, losing out on potential business benefits.

Armin Gerhardt, associate professor of Pharmaceutical Science, Concordia University Wisconsin School of Pharmacy, discusses the effects of moisture on product quality and how to achieve good control of moisture during pharmaceutical manufacturing operations.

The penetrometer PNR 12 from Anton Paar, used for quality monitoring, features the Pharma Qualification Package--Smart PNR 12, which fulfills GMP and United States Pharmacopeia (USP) regulations and can be used to perform qualification processes.

The NEMA 4X Operator Stations, from Ross, Charles & Son, include a 15-inch color touchscreen with Ethernet communications and emergency stop button with hermetically sealed contact block.

The FlexPro 50, from Groninger, is a modular filling and closing system designed to process vials, cartridges, and syringes, as well as vials in bulk and trays.

The Optima AUC from Beckman Coulter Life Sciences is an ultracentrifuge that determines molecular weight, size shape, and polydispersity.

In a move to encourage drug development, EU regulators are offering scientific advice to companies on major efficacy, safety, and quality issues at an early stage.

Click the title above to open the Pharmaceutical Technology May 2016 issue in an interactive PDF format.

The campaign against opioid abuse opens door to more innovative therapies.