
Many pharmaceutical companies are sceptical about IDMP despite the business benefits and its contribution to patient safety.

Many pharmaceutical companies are sceptical about IDMP despite the business benefits and its contribution to patient safety.

The Identification of Medicinal Products (IDMP) standards requires regulatory teams to efficiently collate data from multiple sources and functions.

Learn how to prevent common causes of product loss.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.

Recent legislation and PDUFA initiatives aim to streamline oversight and testing requirements.

Siegfried Schmitt, PhD, Principal Consultant at PAREXEL, discusses how to gain benefits from interactions with industry associations.

CMOs may be gaining as strategic partners to large bio/pharma companies, but they have a much harder path to navigate.
Advances in chemical synthesis are enabling greener, more cost-efficient processes for API manufacturing.

New study will reveal bio/pharma practices and performance on quality issues.

Under US regulations, such as the Medicare Access and CHP Reauthorization Act of 2015 (MACRA), part of any US physician’s reimbursement will be based on patient outcomes.

Value-based medicine is putting patients at the center of pharmaceutical R&D and forcing the industry to move from treatment to prevention.
The author discusses the impact of prefilled syringe product contact materials and the filling and stoppering process on protein aggregates.
Transdermal and inhaled/nasal delivery provide alternative routes of administration for macromolecules.

Solid-phase extraction has several advantages over liquid/liquid extraction for extractables and leachables studies.
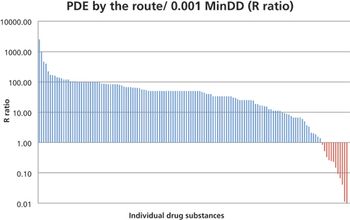
In this study, the authors investigated the relationship between the 0.001 MinDD and the PDE values for 140 drug substances as an attempt to identify high-risk groups of products for patient safety. This comparison can serve as a method for prioritization of APIs for development of PDEs.

The FPB-1P5 sanitary lab mixer is the newest addition to Eirich Machines’ OptimaBlend Fluidizing Blender family of horizontal batch mixing systems.

The portable Flexible Screw Conveyor from Flexicon can be tilted and rolled to serve multiple functions.
The LogTag UTRID-16 Data Logger System from CiK Solutions GmbH includes a built-in USB connector, automatic PDF report generation, and six-level multi-alarm display.

Ross Engineering’s ASME tanks are custom built to serve a wide range of applications and processing requirements.

Click the title above to open the Pharmaceutical Technology May 2017 issue in an interactive PDF format.