
The emergence of new biotherapeutics is both the driver and result of innovative drug development technologies.

The emergence of new biotherapeutics is both the driver and result of innovative drug development technologies.
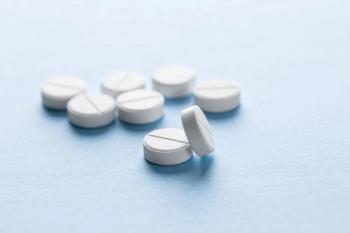
In the present investigation, the fixed-dose combination (FDC) tablet of Atorvastatin calcium and Ezetimibe was prepared by a quality-by-design approach using two-level factorial design.

In addition to ADCs, other types of highly potent biologics require specialized manufacturing skills.
Particle engineering is a vital tool in overcoming many formulation challenges, and technological advances are enabling developers to achieve the full potential of pipeline molecules.

Consider best practices for manual or clean-in-place procedures.

Automation offers benefits for sterile manufacturing in 503B outsourcing facilities.

Process intensification in continuous biopharmaceutical processing can change the way the equipment is used and thus affect cleaning methods.

International pharmacovigilance for biotechs brings about a particular set of challenges, especially for small companies, which face the same rigour as large pharma companies.

As recent COVID-19 vaccine facility citations make clear, failure to meet cleaning and sanitization requirements puts patients, facilities, and operators at risk.

Outsourcing method development offers multiple benefits to companies, including access to experience and expertise, streamlined costs, and development time efficiencies.

Expanded interest in advanced drug manufacturing and continuous production methods calls for more flexible production systems and regulatory policies.

Bio/pharmaceutical manufacturers have made a good first step toward global vaccination through pledging doses at no- to low-profit rates.

Technology advances foster new therapies.

Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, answers some commonly asked questions about regulatory filings.
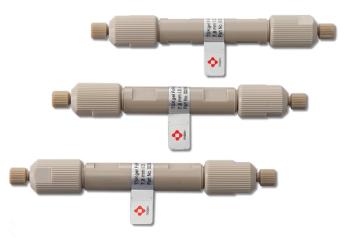
Tosoh Bioscience introduced the TSKgel FcR-IIIA-5PW HPLC column, a semi-preparative affinity column based on a recombinant FcγRIIIA receptor ligand bonded to porous 10 µm polymethacrylate particles.

The TipFil Syringe Filler from TurboFil Packaging Machines is a benchtop syringe filling machine that offers full control of filling parameters in single or dual operation and fills at a rate of up to 12 pieces per minute.

Shimadzu released the TOC-1000e, an analyzer in the eTOC series of on-line TOC analyzers formulated for pure water applications.

The ROSS Double Planetary Mixer from Charles Ross & Son Company is available with an optional weighing system for precise measurements throughout the batching procedure.