
A PQRI expert working group provides case study examples of risk-management applications.

A PQRI expert working group provides case study examples of risk-management applications.

FDA, in cooperation with IPEC, is building a spectral library of excipients to detect improper ingredients within a drug product on site.

The performance of biotechnology venture capital and investment is lackluster at best.

Getting an answer is easy-asking the right question is apparently more difficult.

Editor's picks of new manufacturing products for July 2011.

A new report places pharmaceutical and healthcare companies ahead in corporate and social governance.
GSK Works in Developing Nations to Improve Everything from Research to Roads.

The solid form of an API plays a crucial role in drug quality, and advancing methods for screening, detection, and characterization is key.

Member states in the EU are working to implement the newly passed Falsified Medicines Directive.

Follow-on versions of complex biologics require extensive expertise in development and regulatory procedures.

High demand could lead to innovation in controlled-release injectables.

US support of regenerative medicine is essential for maintaining a lead on healthcare innovation.
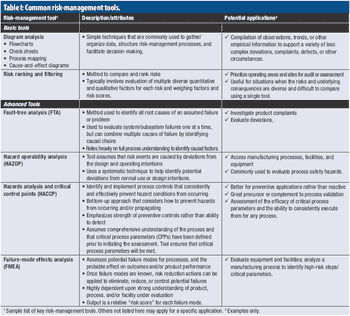
A PQRI expert working group provides case study examples of risk-management applications.

Better strategies and practices in sourcing and procurement can contribute to the bottom line.

Pharmaceutical manufacturers look to various solutions to resolve the challenge of poorly soluble drugs

Pharma's drive for manufacturing efficiency is bolstering particle-characterization technologies.

The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.

Which route will we take to arrive at a national stem-cell policy?

Steve Strickland, general manager of Sensient Pharmaceutical Coating Systems, on recent industry trends.
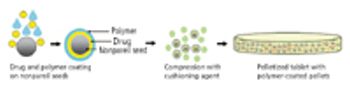
The current review describes the role and selection of excipients, pellet core, coating materials, and compression with various cushioning agents.

Click the title above to open the Pharmaceutical Technology July 2011 issue in an interactive PDF format.