
Agency guidance and industry standards aim to reduce lapses and improve quality operations.

Agency guidance and industry standards aim to reduce lapses and improve quality operations.
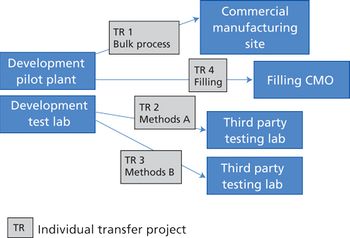
Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.

Innovations protect product quality, improve productivity, enhance sustainability, and simplify usage for patients and caregivers.
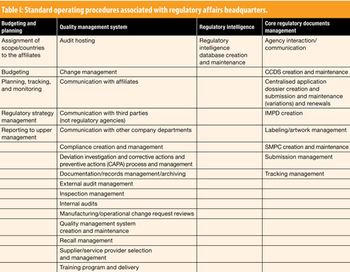
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.

Designing systems using the principles of good documentation practice, including validated audit trails, is a key piece of a manufacturing data integrity program.
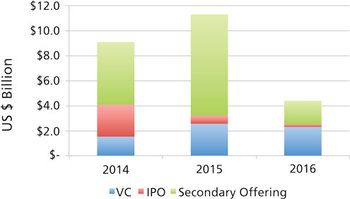
CDMOs need to be aware that unfavorable public markets put emerging bio/pharma R&D spending at risk in 2017.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

A seven-step process may reduce many common tablet and tooling problems, resulting in a better quality end product.

CPhI Pharma Awards seek nominations for excellence in development and manufacturing.

Recent chiral advances demonstrate promise for API synthesis.

The ambr 250 mini benchtop bioreactor system from Sartorius Stedim Biotech can be used for parallel fermentation and cell culture.

The LabX from Mettler Toledo has the ability to connect different laboratory instruments to one management system.

The Double Planetary Mixer (DPM) from Ross, Charles & Son can be used for single-pot mixing and deaeration of viscous formulations such as thick pastes, putties, gels, and other semi-solid products.
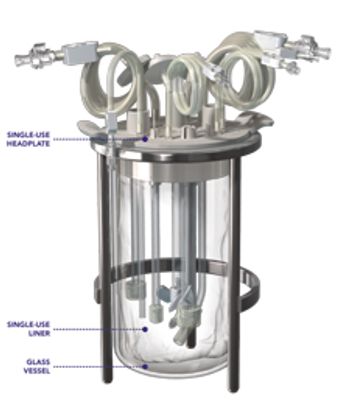
The BIOne single-use bioreactor from Distek is a benchtop scale single-use bioreactor (SUB) system for mammalian cell growth and recombinant protein production.

Polymers have played a key role as solubilizing excipients. Industry experts explain why polymer structures and functionalities are important considerations in formulation development.

Lipid-based drug delivery is increasingly being used to tackle oral bioavailability challenges resulting from poor solubility.

Aseptic spray drying provides an alternative to lyophilization as an enabling stabilization technology for parenteral biologic formulations.

FDA approved the Raplixa, the first spray-dried fibrin sealant, in May 2015 to help control bleeding in adults during surgery.

China and India are also increasing inspections and becoming more exigent about data integrity and cGMPs.

Data integrity and cGMP issues demand closer scrutiny of suppliers. Bribery and corruption may become the next supply chain flashpoint.

The authors' directed isotopic synthesis is one example of how molecular isotopic engineering can be used to predetermine the discrete isotopic ranges of biopharmaceutical products.

The impact of pharmaceutical manufacturing on the environment has triggered demands for tighter environmental controls in EU and national legislations.

Click the title above to open the Pharmaceutical Technology July 2016 issue in an interactive PDF format.