
Contract research, development, and manufacturing organizations invested in new facilities and technologies in 2014 for analytical testing, as well as small- and large-molecule drug development.

Contract research, development, and manufacturing organizations invested in new facilities and technologies in 2014 for analytical testing, as well as small- and large-molecule drug development.

Contract service providers describe how quality by design has influenced a drug sponsor's expectations of suppliers.

Evolving clinical trial research services give biopharmaceutical companies options for full and functional services.

Representatives of contract service organizations that develop biologic-based drugs discussed technology trends such as high-throughput screening and single-use systems.

Experts from contract testing laboratories and service organizations shared their perceptions of analytical testing advances, and challenges still ahead.

Contract services ride high as funding floods bio/pharma.

Representatives of contract service organizations that specialize in parenteral drug development and manufacturing describe the evolving trends.

Despite the growth of specialist companies with capabilities across various therapeutic areas in Europe, there is still a need for early development expertise with end-to-end pharmaceutical manufacturing capabilities.

Contract development and manufacturing organizations identify trends, challenges, and emerging technology and service needs for solid and semi-solid dosage forms.

For a bio/pharma industry in flux, contract services are playing a greater-and more diverse-role in drug development.

Pharmaceutical Technology Europe Marks 25 years of drug-development advances.
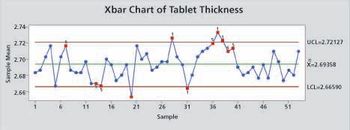
Control charts that are properly constructed and maintained prevent false out-of-control signals and provide a useful method for monitoring a process.