
The pharmaceutical majors target biologics and emerging markets in their manufacturing expansion activities and plans.

The pharmaceutical majors target biologics and emerging markets in their manufacturing expansion activities and plans.
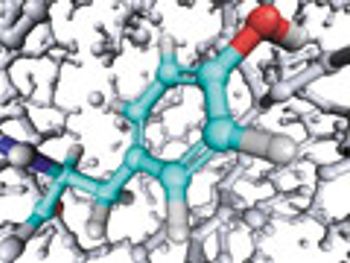
Stapled peptides offer promise to enable cell permeability, binding to therapeutic targets, and modulation of biological pathways.
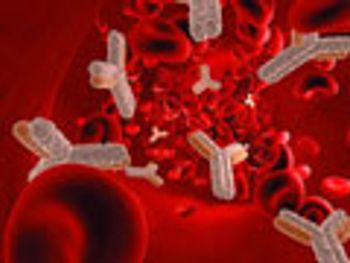
Extensive comparability testing is required to ensure that biosimilars have comparable profiles to their reference products.

The US Supreme Court's Myriad decision satisfied both patient groups and patent holders
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Siegfried Schmitt of PAREXEL explains how regulatory agencies are getting serious about inspections.

Nanomedicines have been authorized by European licensing agencies for more than 30 years but are still posing regulatory difficulties.

Industry players brace themselves to face challenges as India's new drug-pricing policy kicks in full gear.
The authors examine alternative solvents for extractables and leachables screening evaluation of process components that provide extraction equivalence and do not interfere chromatographically.

Editor's Pick's of Pharmaceutical Science & Technology Innovations: The latest in analytical instrumentation including mass spectrometers, calorimeters, and viscometers.

Appropriate use of medications promises to improve patient care and curb spending, prompting pharmacists, public health authorities, and manufacturers to promote multiple adherence strategies.
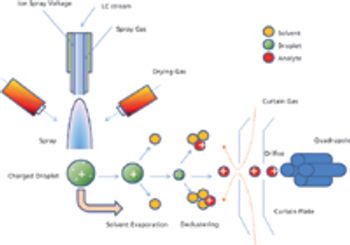
The author describes how liquid chromatography-mass spectrometry works and explains some of its advantages and disadvantages, particularly compared to high-performance liquid chromatography.

The bio-pharmaceutical business outlook in South Korea remains positive.

FDA funds research to further development of innovative generics, while working to address review and approval issues.

As the Supreme Court ruled on generic-drug liability, FDA outlined new rules for warning labels.

Click the title above to open the Pharmaceutical Technology August 2013 issue in an interactive PDF format.