
API's, Excipients and Manufacturing

API's, Excipients and Manufacturing

The authors describe a solid form technology platform used to optimize salt selection, cocrystallization identification and modification, or the development of a free form.

Pfizer has two manufacturing facilities in Germany for high-potency manufacturing, respectively in Freiburg and Illertissen. Pharmaceutical Technology's Executive Editor Patricia Van Arnum visited the facilities and spoke to the company about the design and operation of these facilities.
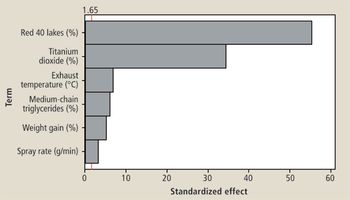
The authors describe a QbD study that was performed to optimize a coating system.

A Q&A with BASF moderated by Patricia Van Arnum.
The authors explain chemical transformations that are achievable through certain biocatalytic routes.

Policymakers must balance fundamental issues involving access to medicines and pricing.

An industry roundtable representing Metrics, Cambrex, Carbogen Amcis, Euticals, Ferro Pfanstiehl, and SAFC.

This article presents an overview of ISPE's guide on project management.

The author discusses the key provisions of GDUFA as they relate to the pharmaceutical supply chain, including parity of inspections between domestic and foreign sites for both finished dosage forms and APIs of generic drugs.