
Combination Drugs - Adding Up the Opportunities in Solid Dosage

Combination Drugs - Adding Up the Opportunities in Solid Dosage
Managing risk in biopharmaceutical operations is of utmost importance for patient protection.

PharmTech spoke with industry experts about the challenges of implementing disposable chromatography systems.
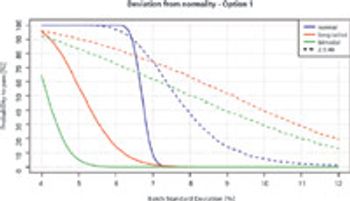
New European Pharmacopoeia chapter aims to resolve problems with applying the harmonized UDU test to large sample sizes.
IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development.

Will the next US President support the backbone of our industry?
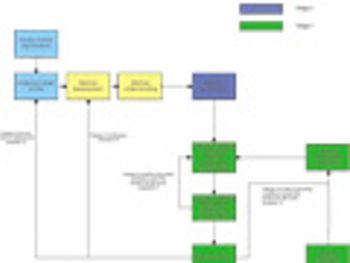
The authors describe how traditional approaches to analytical method and validation may benefit from alignment with quality-by-design concepts.

This article provides a comparison of Rx-360, EXCiPACT and IPEA, available to pharmaceutical manufacturers for the purpose of auditing excipient suppliers and ensuring drug efficacy and patient safety.

Recent research on elucidating the structure and sequence of proteins involves examining the effect of microgravity on protein crystallization and a computational model for protein elucidation.

Potency is a required measurement to determine the amount of active ingredient contained in a preclinical dose formulation. Assessing potency ensures that the test system receives the appropriate amount of active ingredient based on predetermined specifications.

Foreign firms struggle against stricter patent laws, but all is not lost.

Real-time experimentation may offer continuous process improvement.
Today's pharmaceutical companies are striving to reduce costs and maximize efficiencies, and must make decisions on the best way to deploy their limited resources.

Only the strong survive when it comes to pharmaceutical packaging and shipping.

The authors examine the use of various grades of direct-compression mannitol in direct-compression tableting process to evaluate the content uniformity of micronized APIs and excipients in a solid-dosage formulation.

A Q&A with James Ingebrand, Vice President and General Manager of 3M Drug Delivery Systems Division, on recent industry trends.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

New product reviews for October 2012, featuring analytical equipment.

The long-awaited patent cliff that has loomed in the pharmaceutical industry for years has arrived in earnest in 2012, with more than $40 billion in 2011 brand sales facing loss of exclusivity.
This study examines the effect and interaction of variations in hypromellose physicochemical properties.

Working together affords many unseen opportunities for pharmaceutical innovation.
Fixed-dose combination drug therapies give rise to innovation in solid-dosage formulations and manufacturing.

The promise of the Generic Drug User Fee Amendments of 2012 is to end multiyear reviews of new generic drugs and the ever-growing queue of pending applications.

Recent news stories have reported that FDA scientists have been suspected of leaking confidential, commercial, and trade secret information to the media.

Measuring the size of the market for contract manufacturing services requires a careful hand.

Q&A with David Elder and Richard Wright of Strategic Compliance Consulting, PAREXEL International. Both Elder and Wright formerly served with FDA.