
Early-Stage Drug Development

Early-Stage Drug Development

Policymakers must balance fundamental issues involving access to medicines and pricing.

At the inaugural joint FDA/ISPE conference on CGMP earlier this year, CDER Dirctor Janet Woodcock delivered a strong message to the pharmaceutical industry: the efforts to adopt QbD principles must be a top priority.

Manufacturing and in-depth characterization provide basis for demonstrating product equivalence.

The promise of antibody drug conjugates is creating a network of partners among large pharma companies and specialized players.

New product reviews for November 2012.

A Q&A with Yves de Montcheuil, vice-president of marketing at Talend, a provider of open-source integration software.
Consider critical process parameters and strategies to optimize the manufacturing process.

Even when all is well at the facility, one must expect the worst while braving the elements.

The procurement organization rethinks sourcing for maximum efficiency and results.
The European Medicines Agency has added granularity to its biosimilars approval pathway by releasing a guideline on biosimilar monoclonal antibodies (mAbs).

Regulatory bureaucracy in Europe coupled with the demand for lower-priced medicines continues to hinder efforts in innovation for Alzheimer's disease.
Pharmaceutical companies and contract service providers adapt strategies and capabilities to reduce costs and accelerate drug-development timelines.

Experts share insight into extractables and leachables testing, including high-risk products, analytical testing, and regulatory requirements from FDA and EMA.

A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.

Key talks from the recent PDA/FDA regulatory conference highlight room for improvement.

The government of Turkey is drawing up a program in coordination with the pharmaceutical industry to create ways to make the country a regional production center for pharmaceuticals serving Europe, Central Asia, and the Middle East.
To support global stability practices, fundamental basics must be in place.

This article introduces"mean kinetic relative humidity" for evaluating the impact of humidity variability.
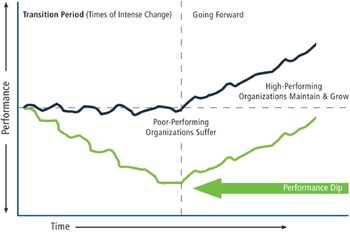
A disciplined approach to changing behavior can achieve change agility.

Overt and covert packaging technologies evolve to authenticate drugs and fight counterfeits.

The US Pharmacopeia's revised General Chapters on elemental impurity limits and testing procedures are set to take effect in December 2012.

Global tactics that incorporate online technologies and social media are reshaping disease response.

The Pharmaceutical Inspection Co-operation Scheme (PIC/S) has finalized a risk-based inspection planning tool for inspectorates to use in applying science- and risk-based principles to planning GMP inspections.