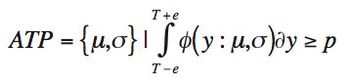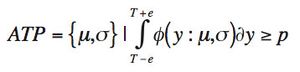Articles by Stephen Colgan
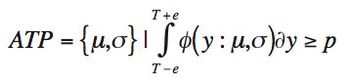
Using Quality by Design to Develop Robust Chromatographic Methods
ByStephen Colgan,Jackson Pellett,Melissa Hanna-Brown,Gregory Sluggett,Tim Graul,Loren Wrisley,Gregory Steeno,Jim Morgado,Roman Szucs,Brent Harrington,Kimber Barnett The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

Pharmaceutical Stability: Scientific and Regulatory Considerations for Global Drug Development and Commercialization
BySteven W. Baertschi, PhD,Mark Alasandro,Stephen Colgan,Saji Thomas,Dilip R. Choudhury,Bekki Komas,Robert H. Seevers,John Bobiak,David Lin,Robert J. Timpano,Ramesh K. Sood,Ganapathy Mohan,M. J. Skibic,Brian W. Pack,Kim Huynh-Ba A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.

AAPS Workshop Summary
BySteven W. Baertschi, PhD,Mark Alasandro,Stephen Colgan,Saji Thomas,Dilip R. Choudhury,Bekki Komas,Robert H. Seevers,John Bobiak,Ramesh K. Sood,M. J. Skibic,Brian W. Pack,Kim Huynh-Ba A two-day workshop on the "science behind pharmaceutical stability" was held in conjunction with the Annual Meeting of American Association of Pharmaceutical Scientists (AAPS) on Oct. 21-22, 2011 in Washington, DC.
Early Development GMPs for Stability (Part IV)
ByQ. Chan Li,Z. Jane Li,Tony Mazzeo,Robert Timpano,Mark Alasandro,Stephen Colgan,Andy Rignall,Z. Jessica Tan,Frank Diana,Bruce Acken,Paul Curry IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development, with a focus on stability.