
Pharma companies are concerned they may have to postpone plans for the commercialization of new combination products because of delays in obtaining marketing authorizations because of a lack of clarity about approval rules.

Pharma companies are concerned they may have to postpone plans for the commercialization of new combination products because of delays in obtaining marketing authorizations because of a lack of clarity about approval rules.

A matrix of multi-functional cleanrooms can be adapted for launching products.

Mechanistic process and product modeling turns data into knowledge that can be extrapolated with known confidence for design space characterization and risk assessment.

Wilden, part of PSG, a Dover company, released its new 6 mm (1/4”) Velocity Series air-operated double-diaphragm (AODD) pump.

Ross, Charles & Son’s Laboratory Paddle Blender offers advanced features for automated powder blending and liquid spraying operation with recipe management.

Ross, Charles & Son added a custom 150-gallon Triple Shaft Mixer, the Ross VersaMix Model VMC-150, with elaborate automation and safety functions.

Stream Bio’s Conjugated Polymer Nanoparticle products are a new generation of novel molecular bioimaging probes for diagnostic and therapeutic applications.

Building up relevant expertise in-house will make writing spec sheets for software easier, according to Siegfried Schmitt, principal consultant at PAREXEL.

The growth in adoption of single-use systems for commercial manufacturing will be dramatic in coming years.
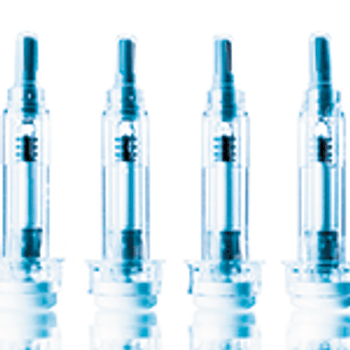
As biologic drugs grow increasingly complex, drug delivery mechanisms such as prefilled syringes are being adapted to meet the challenges.
This article will explore the requirements for media and supplements needed to maintain newer cell lines, such as those based on human cells and fungal cells.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.

Demand for highly potent APIs continues to rise.

Survey results and record attendance show positive signs for various bio/pharma regions.

Experts share best practices, and war stories, for a crucial but often underappreciated part of drug development.

New FDA guidance offers a time buffer but increases supply-chain-management complexity, as active DSCSA enforcement begins.

Click the title above to open the Pharmaceutical Technology November 2018 issue in an interactive PDF format.

… Brexit may be a bumpy ride. Here, the new European editor of the PharmTech Group, Felicity Thomas, discusses Brexit and its implications for the pharma industry.

To achieve a more dynamic marketplace, FDA is issuingguidance documents and targeted advisories to support R&D on complex generics and combination products.