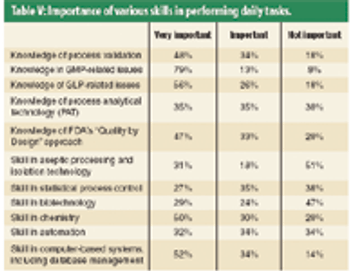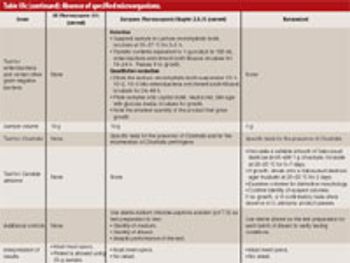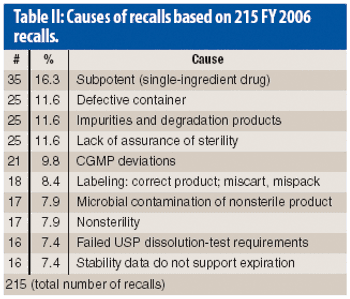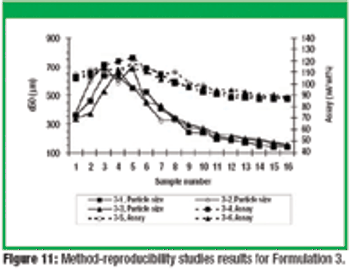
This article describes the design and development of a material-sparing fluidization segregation tester for use with pharmaceutical powders. This tester offers several improvements over the current ASTM standardized test practice. Less than 20 mL of material is required to characterize the fluidization segregation potential of a sample. Features of the tester include powder containment for potent compounds, in-process monitoring of the fluidization conditions, and sample retrieval without the need for subsampling or riffling for typical analyses.