
Global Healthcare on the Ground: USAID Works to Carry out President Obama's Global Health Initiative
Cultivating a productive investment environment will require partnerships with a range of stakeholders.

Cultivating a productive investment environment will require partnerships with a range of stakeholders.
Expansion activity was limited as fine-chemical producers and CMOs of API and intermediates grapple with changing industry fundamentals.

A dearth of late-stage candidates could hurt the pharmaceutical services market in the future.

Cleanliness is crucial, even if zapping and trapping is necessary to reduce product contamination.

The seventh in a series of eight case studies from the Product Quality Research Institute focuses on packout remedies.

As the excipient supply chain becomes more complex, industry must up the ante to comply with new standards and regulations.

Added responsibilities and outside concerns prompt overhaul of agency's structure.

New product reviews for December 2011, focusing on manufacturing.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

Precedents set in the historic Barr case continue to raise questions over suitable sample-size criteria.

Political leaders need to consider the impact of the biopharmaceutical industry on the economy.

Packaging is indeed headed to be a lead sector in the Asian pharmaceutical environment, but certain challenges must first be overcome.

A Q&A with Deborah Tanner, executive vice-president and group president of R&D laboratories at Covance, on recent industry trends.

The authors developed a metronidazole-based floating drug-delivery system to investigate the effect of rate-controlling polymers on release pattern and duration of buoyancy in matrix tablets.

Readers react to the economic turmoil of the past year and look longingly forward to 2012.

Pharma companies must balance demand for new drugs while facing reduced R&D spending.

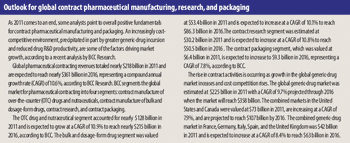
As 2011 comes to a close, a new paradigm of product development is ever more important.

A new book attempts to reduce the confusion and costs associated with regulatory compliance.

Employee training-at all levels-is crucial for moving forward with a successful risk- and quality-based manufacturing strategy.

The last in a series of eight case studies from the Product Quality Research Institute focuses on internal GMP audits.
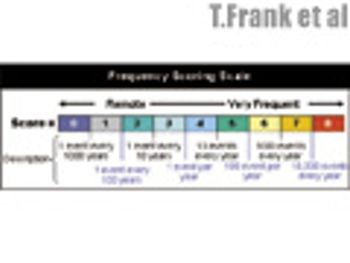
The sixth in a series of eight case studies from the Product Quality Research Institute focuses on packaging line GMP optimization.

Click the title above to open the Pharmaceutical Technology December 2011 issue in an interactive PDF format.