
Evolving industry demands are driving the need for greater flexibility and innovation in processing equipment for pharmaceutical manufacturing.

Evolving industry demands are driving the need for greater flexibility and innovation in processing equipment for pharmaceutical manufacturing.

Pharmaceutical training programs are enhanced by integrating ICH quality risk management considerations.
Automation, miniaturization, and new software algorithms are improving throughput and accuracy.

Shifting to automated, closed modular manufacturing systems is growing increasingly crucial for biopharmaceutical production.

Capturing and curating R&D data are crucial to realizing the full value of advanced analytics.

The complexities of tech transfer may be overcome by data-driven approaches, digital tools, and effective communication.

Recent hurricanes in the US close Baxter plant, shining a spotlight on supply chain fragility again.

The product license holder must have a supplier oversight system in place, says Siegfried Schmitt, PhD, vice president, Technical, Parexel.

The ROSS FDA-50 Fixed Tank Dual Shaft Mixer is built to handle a wide range of formulations and viscosities and is ideal for processes that require meticulous control over mixing, temperature, and pressure.

The Mobius ADC Reactor is the first single-use reactor designed specifically for the manufacture of antibody drug conjugates.
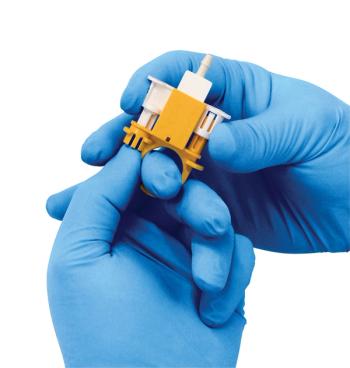
CPC’s MicroCNX ULT Series represents the first aseptic microconnector that can be fitted directly into freeze cassettes for GCT production.

The new cassette offers more efficient and sustainable ultrafiltration and diafiltration processes for feed volumes of 100 mL–1000 mL.