
PTSM: Pharmaceutical Technology Sourcing and Management
GSK and Save the Children announce criteria for awards that recognize innovation in healthcare for the world&s poorest children.

PTSM: Pharmaceutical Technology Sourcing and Management
GSK and Save the Children announce criteria for awards that recognize innovation in healthcare for the world&s poorest children.

PTSM: Pharmaceutical Technology Sourcing and Management
Covance MarketPlace is designed to enable partnerships between the company';s emerging biotechnology and established pharmaceutical clients.

PTSM: Pharmaceutical Technology Sourcing and Management
PharmSource special report shows demand for cytotoxic injectable drugs could tap existing CMO capacity.

PTSM: Pharmaceutical Technology Sourcing and Management
Pharma companies are looking to external sources for innovations that meet future needs.
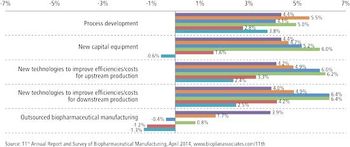
PTSM: Pharmaceutical Technology Sourcing and Management
With budgets growing, clients see CMOs' costs as less crucial and consider other factors.

PTSM: Pharmaceutical Technology Sourcing and Management
Cytovance Biologics completes GMP manufacture of ARMO BioSciences& lead product.

PTSM: Pharmaceutical Technology Sourcing and Management
Regulators and industry organizations explain policies and standards to manufacturers and authorities across the globe.

PTSM: Pharmaceutical Technology Sourcing and Management
Aesica Pharmaceuticals S.r.I. collaborates with QAD to meet China&s Food and Drug Administration's shortened serialization deadline.

PTSM: Pharmaceutical Technology Sourcing and Management
Frontage Labs will expand its CMC facility in Exton, PA.

PTSM: Pharmaceutical Technology Sourcing and Management
Novartis' 13th Annual Malaria Expert Panel focused on access to quality anti-malarials across Africa.

PTSM: Pharmaceutical Technology Sourcing and Management
Rentschler plans to build two stainless-steel bioreactors to target market supply.

PTSM: Pharmaceutical Technology Sourcing and Management
Medicenna Therapeutics selects Kalon Biotherapeutics to manufacture a new brain cancer drug for children and adults.

PTSM: Pharmaceutical Technology Sourcing and Management
Recipharm and CTC will offer packages for Phase I studies.

PTSM: Pharmaceutical Technology Sourcing and Management
PhRMA announced 430 new medicines in development to treat chronic diseases affecting older Americans.

PTSM: Pharmaceutical Technology Sourcing and Management
SGS introduces the Cobas 6000 Analyzer System for its France-based laboratory.

PTSM: Pharmaceutical Technology Sourcing and Management
Jones Packaging introduces a new solid dose vial line in its Toronto facility.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA cites cGMP violations for API manufacturing at a facility in Tianjin, China.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA inspection found cGMP violations in the Bangalore, India API manufacturing facility.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA provides advice to supply-chain stakeholders on how to identify suspect drug products and how to notify the agency of those products.

PTSM: Pharmaceutical Technology Sourcing and Management
Industry seeks alternatives to costly and toxic precious metals used to form the catalysts for organic synthesis.