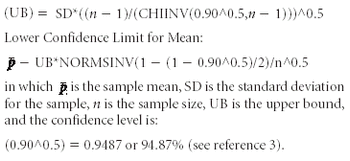
Using the Bergum Method and the MS Excel software program, the author determines the probability of passing the USP dissolution test.
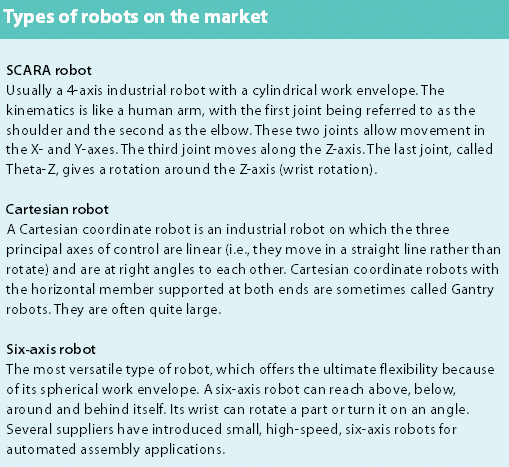
There is a rapidly growing ageing population that requires sophisticated medical devices and newer drugs. This is likely to result in an increase in the use of robotics to improve manufacturing efficiency. This article looks at the role of SCARA robots in pharmaceutical plants and laboratories.

Using the Bergum Method and the MS Excel software program, the author determines the probability of passing the USP dissolution test.
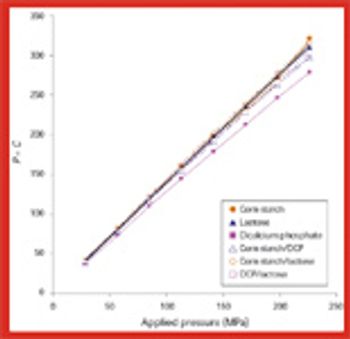
The survival of Bacillus subtilis spores in dicalcium phosphate, lactose, and corn starch and in their binary mixtures depends on the compressional properties of these materials and on parameters involved during the tableting process, including compression speed.

The attraction of nasal administered therapeutic agents is obvious, including faster onset of action, increased compliance and avoiding degradation during first pass metabolism.
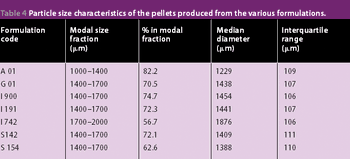
The previous studies on the incorporation of glyceryl monostearate into pellets by extrusion/spheronization has been extended to include a range of grades of this material plus a mixed medium chain partial glyceride and two glycerol esters of hydrogenated natural glycerides as described in this article.
To produce an application or solution for a specific domain, a vendor must demonstrate a thorough understanding of the domain and its specific challenges.
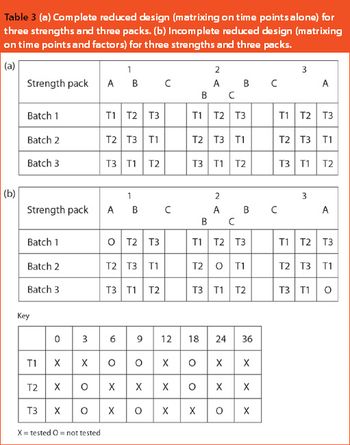
This article looks at the use of bracketing and matrixing to lower the number of stability samples required and, consequently, reduce the cost of sample production, testing and management. There is a common misconception that regulatory authorities will not accept such methods, but there is actually an International Conference on Harmonization guideline (ICH Q1D) on the subject. In fact, many of these designs have already been accepted and FDA members were among the first to describe matrixing.
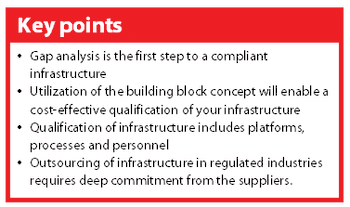
A new Good Automated Manufacturing Practice (GAMP) guide on IT Infrastructure Control & Compliance was launched in Chicago (IL, USA) 23 August 2005.1 The guide is intended to support pharmaceutical companies in their effort to establish a well-defined and compliant infrastructure. This article discusses different aspects of the guide that may support your organization in getting — and keeping — your infrastructure under control.

GSK Study Questions Bioequivalency of Generic Cold Sore Creams

Formulators currently face numerous challenges in nanosuspension development in terms of ensuring safety, efficacy, and stability. Presenters at Wednesday's AAPS symposium offered strategies for addressing these challenges, including setting meaningful particle-size specifications, selecting the method to measure particles in nanosuspensions (especially for nonspherical particles), gaining a meaningful particle-size distribution, and determining the particle size from such distributions.

As a pharmaceutical formulation tool, molecular simulation is currently in its early infancy. Nonetheless, presenters at Wednesday?s AAPS Annual Meeting and Exposition demonstrated that the technology is beginning to attract some interest. The topic was discussed in a presentation titled "Application of Molecular Simulations to Formulation Development and Stability Prediction."

Artium Technologies' (Sunnyvale, CA, www.artium.com) new diode-pumped phase Doppler interferometry systems use solid-state lasers incorporated into transmitting optics, eliminating losses that can result from fiber coupling, alignment, and degradation. According to Atrium, the advantage of this approach to optical design is improved precision and a larger dynamic range, with higher resolution over the entire range.

In the spirit that a good review of the fundamentals is always beneficial, the American Association of Pharmaceutical Scientists' Annual Meeting and Exposition featured an early morning discussion about the basic aspects of dissolution testing, including common sources of errors and deviations. The well-attended session proved that dissolution testing remains a topic of interest, especially as the industry continues to extend its application to media other than solid dosage forms, most notably soft gels.
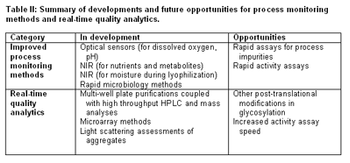
"The better we understand the relationship between process parameters and product attributes, the better control we'll have over product quality," said Beth Fowler, PhD, during Tuesday?s session on process monitoring at the AAPS Annual Meeting.

When it comes to developing a robust lyophilization process, formulators can "pay now or pay later," says Jeff Schwegman, PhD, founder and chief scientific officer for BioConvergence. Because 30% of new drugs in clinical trials are biotech-based therapeutics (compared with 7% 10 years ago), more than ever, the US Food and Drug Administration is paying close attention to lyophilization data and questioning pharmaceutical companies about their development cycles, especially cycle development transfer, shelf-temperature mapping, dryer-to-dryer comparison studies, formulation time, process validation, and cycle deviation. Consequently, this is pushing formulators to optimize formulation variables, conduct additional testing during early-stage development, and understanding critical process parameters, equipment qualifications, and manufacturing conditions that can influence formulation behavior at a large scale. Not taking the time or effort to achieve these goals during early development could lead to redundancies in formulation work - a reality observed too often in today's practices.

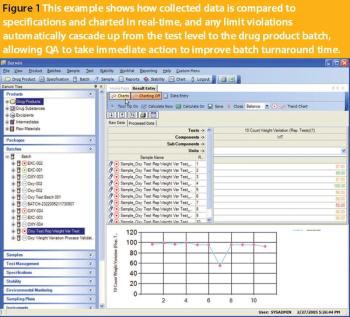
To implement process analytical technology systems into the current information technology landscape, manufacturers will need to adopt continuously available systems and infrastructures.

Although there is no global regulation or industry standard on labelling requirements, some organizations are beginning to lay down their own standards.
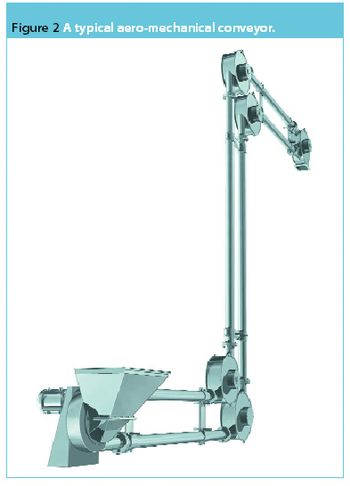
The EU ATEX Directive 1999/92/EC (ATEX 137) regarding the minimum requirements to protect the health and safety of workers potentially at risk from explosive atmospheres came into European law in January 2000. In the UK, the ATEX 137 Directive has been implemented as part of the Dangerous Substances and Explosive Atmospheres Regulations (DSEARs), which were issued in December 2002.

Process industries are faced with increasing demands for product safety, improved quality, efficiency and profitability. The chemical, pharma and cosmetic industries are no exception.

Virtual labels eliminate the need for troublesome transfer ribbons or inkjet fluids, which are inherent with traditional labelling technologies.

Biomanufacturing managers believe the current lack of adequately trained personnel is one of the most serious problems facing the biomanufacturing industry.
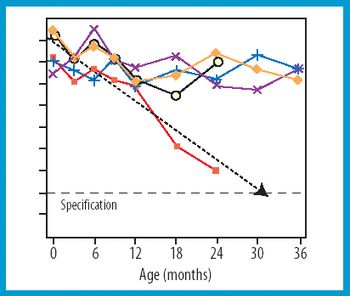
In a follow up to its 2003 partner article, the authors suggest three different levels of out-of-trend stability data and discuss issues surrounding the identification and investigation of each level.
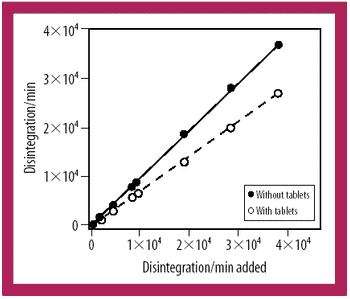
A radiotracer technique is a simple, fast, and sensitive technique for analyzing the integrity of clinical supply packages to water.
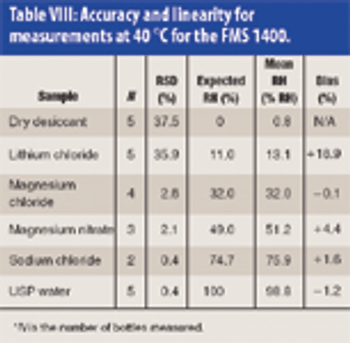
Frequency modulation spectroscopy is a nondestructive technology for determining the water activity of pharmaceutical samples. This article discusses the various pharmaceutical applications of frequency modulation spectroscopy, offers comparisons with various traditional water activity measurement techniques, and presents an assessment of various instrument performance elements.

Two scenarios demonstrate the need to use the percent of parent drug loss rather than the percent of degradation products formed when reconciling mass balance calculations.

Chemical purity is the most important quality characteristic of a pharmaceutical substance. This article describes the latest scientific and technological advances to meet recent pharmacopoeial and regulatory requirements regarding the control of organic impurities in synthetically produced active substances. Future developments and suggestions for those working in quality control and raw material selection are discussed.