
Contract manufacturers serving the biopharmaceutical market add capacity in a bet on renewed growth for biomanufacturing.

Dust extraction and centralized vacuum cleaning systems vary in their design, performance and costs. Different companies have different approaches to their design, however, there are some basic rules that must be followed if these systems are going to be immediately effective and avoid future problems.

Contract manufacturers serving the biopharmaceutical market add capacity in a bet on renewed growth for biomanufacturing.
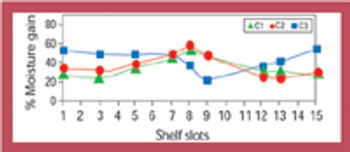
Stability data that do not follow the expected trend in comparison with other stability batches or previous results collected during a stability study are considered out-of-trend (OOT) results. OOT stability results recently have gained the attention of regulatory agencies and as a result, the approach for identifying and investigating OOT results has become a topic of increased discussion. One example is a 2003 article by the PhRMA Chemistry, Manufacturing, and Controls Statistics and Stability Expert Teams, which was intended to initiate dialogue on how to address OOT results (1).

The first part of this article introduced the basic features of Raman spectroscopy and presented some examples of its application in the pharmaceutical industry. This second part focusses on the technique's application as a PAT tool within the pharmaceutical manufacturing environment. FDA's PAT initiative has provided motivation to explore the application of 'new' analytical technologies to the pharmaceutical manufacturing process and Raman spectroscopy shows great promise. The strengths and weaknesses of the technique as a potential PAT tool are discussed together with some examples of how this works in practice in a pharmaceutical manufacturing environment.


In his Feb. 2005 viewpoint article, "In Defense of Singlet Testing," Torbeck (1) draws an important philosophical distinction between "standards" and "specifications." He argues that specifications are criteria selected by manufacturers for statistical control of their products, whereas compendial standards are absolute requirements. This distinction is entirely compatible with modern concepts of statistical process control.

Detection and identification of different polymorphic forms is, therefore, important throughout the drug development and manufacturing process.
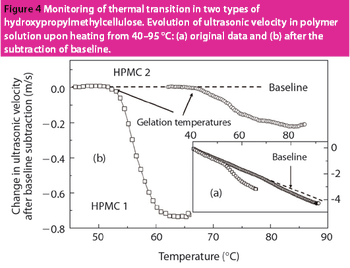
HR-US is a nondestructive technique with enormous potential for the analysis of materials and formulations used in the pharmaceutical industry.

Possible cross-contamination issues should be eliminated at the early stage of the project. The project sponsor should ensure that all relevant personnel from the production, quality control, logistics, and maintenance departments, as well as engineering, are involved in the conceptual stages of a design.
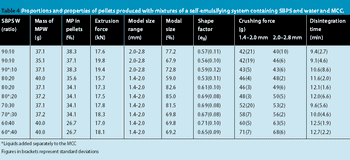
This high quality of pellet roundness is surprising in view of the high extrusion forces required to extrude the wet masses.

Glossary of computer and software terms.

The use of quality management software (QMS) to automate manufacturing quality processes quickly is becoming an industry-wide initiative. Companies are turning to commercial off-the-shelf systems (COTS) to simplify implementation and validation efforts. Regardless of the system selected, the automation of these critical quality processes is subject to electronic records and electronic signatures (ER/ES) requirements, as set forth in the 21 CFR Part 11 regulation (1). Therefore, companies must adopt a comprehensive but manageable approach to Part 11 compliance as they begin to automate the processes that support product quality and efficacy to ensure they meet the requirements of good manufacturing practices and all predicate rules.

New Chemical Reaction Method Accelerates Indole Production

A newly developed software program transforms written SOPs for all required analytical method validation experiments into transferable automated templates, integrating individual activities and technologies under one platform.

Asieve or screener is an essential part of every pharmaceutical production process, particularly as product quality and integrity are so important. The use of a sieve gets rid of oversized contamination to ensure that ingredients and finished products are quality assured during production and before use or despatch.

The FDA initiative —Process Analytical Technologies (PAT) — is slowly gaining momentum, creating a revolution in manufacturing and testing processes that aims to ensure product quality. Its growth will encourage faster testing techniques to bring analytical testing closer to on- and at-line testing during the product manufacturing process.
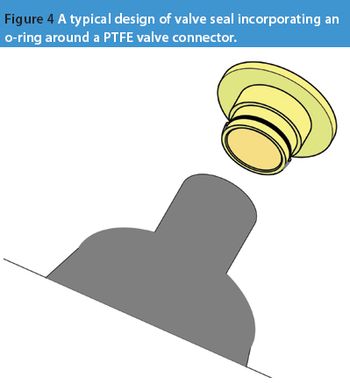
Active pharmaceutical ingredients (APIs) have become more potent, therefore the requirements of good manufacturing practice (GMP) are making ever more stringent demands on valve design and sealing. An absence of dead space, ease of cleaning and flushing is the norm for valves where cross contamination must be avoided at all costs. Sealing valves to glass reaction vessels has lagged behind valve sealing for steel vessels.

The authors propose a strategy for classifying and validating inprocess testing methods.

The author proposes a new analytical graphic, the sector chart, which presents data that cannot be adequately presented with current graphs.This chart combines features of zone charts with the basic principle of precontrol charts. It addresses engineering control and is superior for representing data such as beneficial and adverse trends.

The validation of alternative microbiological testing is an opportunity for a manufacturer to decrease the amount of time required for laboratory results.To properly validate these alternatives, a practitioner must first identify what is being studied. The regulatory effect on established product and process specifications and levels must be completely evaluated, as changing the method of analysis may well change the pparent number in the sample.

The C-SOC team must develop a network of committed industrial partners who will participate in the direction, execution, and evaluation of research and educational activities.

X-ray microtomography has great potential for improving the understanding of the structural features of solid dosage forms and the changes in those features during manufacturing, handling, and storage.This article describes the basic principles of the technique and provides examples of its potential applications.

A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and assess the precision of each device.

Nanotechnology is believed to hold enormous promise for the future of medicine and healthcare...
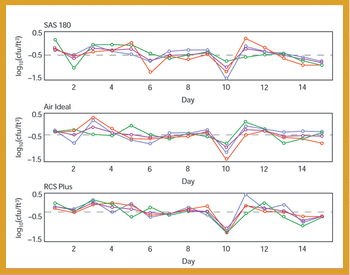
A comparative study of three air samplers used for bioaerosol collection was performed to evaluate the average recovery of colony-forming units and to assess the precision of each device.

Nanostructured lipid carriers are a new type of delivery system offering improved performance in terms of drug loading and long-term stability with the ability to form highly concentrated dispersions...