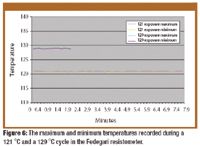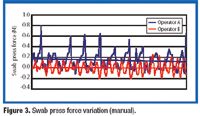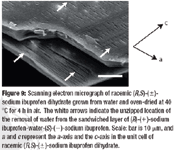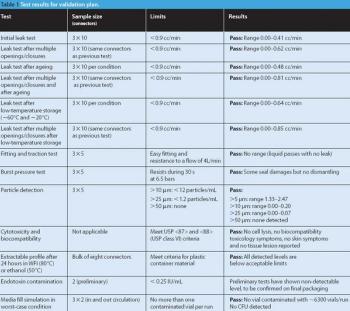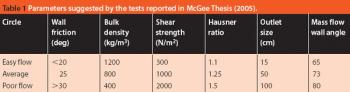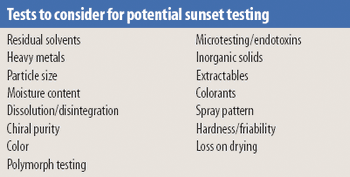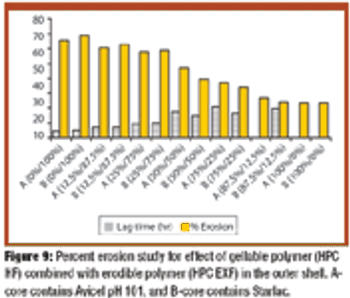
Fluidized hot melt granulation (FHMG) is an emerging technique combining the advantages of both dry and wet granulation methods, and represents an innovative continuous granulation process capable of mixing and agglomerating excipients and active pharmaceutical ingredients (APIs) to produce uniform blends of particles suitable for use in the manufacture of pharmaceutically elegant solid dosage forms.