
As public confidence in the drug development process waivers, leading vaccine developers promise to adhere to scientific and regulatory principles.

As public confidence in the drug development process waivers, leading vaccine developers promise to adhere to scientific and regulatory principles.

Rapid scale-up to billions of doses requires collaborative, all-out efforts by innovators, their manufacturing partners, and the entire supply chain.

Tight development timelines and accelerated approval pathways favor simple, cost-effective capsule formulations.
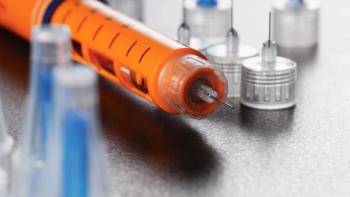
A device manufacturing process must be carefully designed in the early stages of development to ensure success in commercial manufacturing.

End-user considerations are becoming increasingly important as they can provide a lot of value and help to ensure commercial success of a drug.
Early adopters and equipment manufacturers refine their equipment and processes, paving the way for broader use.

Catalent will manufacture drug substance for AZD1222 at its Harmans, MD facility.

EC finalizes agreement to purchase millions of doses of AstraZeneca's COVID-19 vaccine.

CARBOGEN AMCIS has announced significant investments to increase manufacturing capacity in Switzerland and France.

Novavax has signed an agreement with the UK government for 60 million doses of a COVID-19 vaccine and a Phase III clinical trial.

Plastic primary packaging could help prevent supply chain shortages associated with high demand for COVID-19 vaccines.

Fujifilm Diosynth Biotechnologies’ North Carolina site will be used to manufacture Novavax’ NVX‑CoV2373 vaccine candidate for a Phase III clinical trial.

Study reports immune and T-cell response from CanSino COVID-19 vaccine candidate.

Preliminary data from a German Phase I/II trial shows Pfizer/BioNTech COVID-19 vaccine candidate produces immune response.

Strong immune response by patients receiving two doses of vaccine suggests a possible treatment strategy.

Data and software identify optimum pharmaceutical packaging choices for the required shelf-life.

Demand for custom drug delivery solutions is increasing and bringing forth an exciting period of valuable, innovative development opportunities.
Controlled-release formulations offer numerous advantages for developers and patients, and this market is expected to continue to experience growth in the near future.

Using a specific addition method to wet the powder bed in twin-screw granulation using a foam binder improves robustness for continuous solid-dosage drug product manufacturing.
RNA is easier to manipulate than DNA but challenging to deliver to the right cells.

EMA’s Committee for Human Medicinal Products (CHMP) has recommended that Kaftrio, a triple combination therapy for the treatment of cystic fibrosis, be authorized for marketing in the European Union.

The European Medicines Agency (EMA) has recommended a conditional marketing authorization be granted for Idefirix (imlinfidase) for the treatment of highly sensitized adult patients waiting for a kidney transplant.

The continuous manufacturing platform can be used for individual unit operations or a complete solid-dosage manufacturing line and can be integrated with third-party equipment.

Full results from the Phase III ETHOS trial have demonstrated a statistically significant reduction in the rate of moderate or severe exacerbations in patients with moderate to very severe COPD after treatment with triple-combination therapy Breztri Aerosphere.

Inovio received a $71-million contract from the US Department of Defense to scale up manufacture of its Cellectra smart device to deliver its DNA vaccine.