Engineering nanoparticles with optimal properties for use in cancer therapies.

Engineering nanoparticles with optimal properties for use in cancer therapies.

Visual mapping can provide a particle-size distribution estimate.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.
Pharmaceutical Technology brought together a panel of industry experts for a special forum to discuss solubilizing polymers and the related formulation strategies for poorly soluble drugs.

Capsugel’s acquisition of Encap Drug Delivery expands its lipid expertise and adds an FDA-inspected commercial manufacturing facility.

The tableting science anti-research (TSAR) project seeks to understand why certain formulations stick to tablet tooling.

Factors for assessing excipient variability, the associated challenges developers need to address to design and manufacture solid oral drug products, and solutions for such challenges are examined.
The use of various documents and guides by the International Pharmaceutical Excipients Council can facilitate the flow of information among excipient manufacturers, distributors, and users.
A roundtable discussion of the challenges and innovations in tablet splitting featuring Freeman Technology, Accu-Break Pharmaceuticals, and Medelpharm.

Specially designed excipients, improvements in processing capabilities, and a growing understanding of the hot-melt extrusion (HME) process is increasing the use of HME as an approach for enhancing the bioavailability of poorly soluble drugs.

A GBI Research report says the opioid market will be driven by development of new products and post-marketing studies designed to reduce abuse risk.
As contract API manufacturers, fine-chemical producers, and pharmaceutical companies prepare to attend Informex in Anaheim this month, the state of the industry shows improving conditions in certain sectors.

Siegfried Schmitt, a principal consultant with PAREXEL, discusses how the EU's Falsified Medicines Directive will affect US API production.

When speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.
Process chemists employ a variety of approaches to improve yield, purity, and stereoselectivity.

The agency recommends to avoid the use of to dibutyl phthalate and di(2-ethylhexyl) phthalate in CDER-regulated drug and biologic products, including prescription and nonprescription products.

GlaxoSmithKline recently developed a novel technology for the formulation of modified-release tablets. The authors describe the route from development to commercialization.
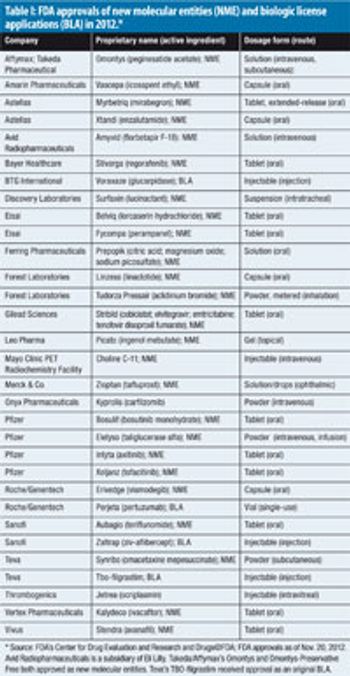
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

Novartis announced that it has received FDA approval for a seasonal influenza vaccine produced in cell culture, the first seasonal vaccine produced by this method to be approved in the US.

Boehringer Ingelheim has entered into a non-exclusive technology and commercial license option agreement with BaroFold. Under the terms of the agreement, Boehringer Ingelheim will license BaroFold's pressure-enabled manufacturing technology (PreEMT).

German Court Finds AstraZeneca's Seroquel XR Formulation Patent Invalid; FDA Advisory Committee to Recommend Approval of GSK's H5N1 Influenza Vaccine Candidate; and More.

The promise of antibody drug conjugates is creating a network of partners among large pharma companies and specialized players.
The authors describe a process for generating high affinity, fully human antibodies in culture.
This article focuses on the growing need for effective data management in the life sciences industry-especially among smaller pharmaceutical manufacturers.
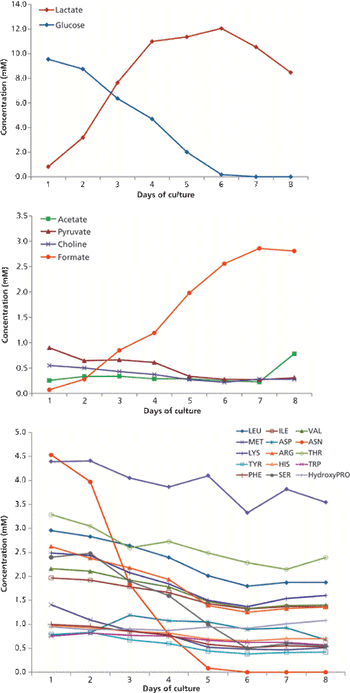
The author discusses current expectations in bioprocessing and lays a framework for using NMR to enhance a QbD approach.