
FDA modernizes information systems, expands access to drug safety and use information.

FDA modernizes information systems, expands access to drug safety and use information.

From last-minute product inserts to putting out fires, close calls are a common occurrence.
Technological developments make it easier to manufacture sterile parenterals.


FDA issues data on drug-induced liver injuries and serious skin reactions; IPEC Federation Week starts in France.

Liquids are used in a wide variety of industries, either during manufacture or as end-products in themselves. Whatever the end product, quality control of liquids is crucial to many industries.

The European Medicines Agency and the Swiss Agency for Therapeutic Products, Swissmedic, have agreed to exchange information about the authorization and safety of H1N1 pandemic medicines.

Margaret Hamburg, commissioner of the US Food and Drug Administration, unveiled a new program to improve the efficiency of import inspections.

EMA statistics highlighting centralized-procedure activities for human medicines show a significant increase in positive opinions made between 2007 and 2009.

FDA Releases Annual Guidance Agenda; OMB Urges Obama to Issue Revised Executive Order.

Less than a week after president Obama proposed a spending freeze on nonsecurity-related federal programs, the US Food and Drug Administration released its fiscal year 2011 budget request, calling for a 23% increase.

Cephalon Buys Mepha; BASi's CEO Retires; and More.
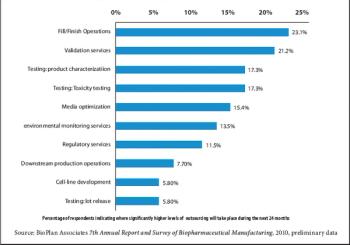
Strategic rather than tactical considerations are driving biopharmaceutical outsourcing.

Sharing too much-or too little-information can have disastrous onsequences.

FDA impersonators and counterfeit drugs threaten the public's trust in online pharmacies.

Vaccine R&D is surging, but continues to raise manufacturing and regulatory challenges.

Regulators and industry move to require inspections of API manufacturing facilities.
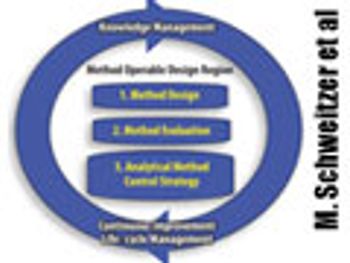
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods. This article contain bonus online material.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.
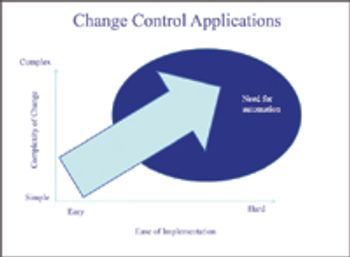
We never thought implementing complex changes could become more cumbersome.

The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods.

Although representing a huge market, the EU also presents problems that hamper profitability- particularly when it comes to differing price regulations across member states.

Pharma faces imminent expiries of key patents and increasing competition from generics. To protect what they already have, or what they will invent or acquire, companies must give patent protection the attention it deserves.

References for the article published in the February issue of Pharmaceutical Technology Europe.

New York Governor Wants Stricter Limits On Pharmaceutical Marketing; And More.